Overview
The article underscores the ten key benefits of employing silver electrodes in pharmaceutical laboratories, asserting their precision, stability, and cost-effectiveness as pivotal elements for enhancing research outcomes. By detailing advancements in silver electrode technology, it illustrates how these innovations bolster performance and reliability—critical factors for achieving accurate measurements in vital pharmaceutical applications. This exploration not only captures attention but also builds interest in the transformative impact of high-quality scientific instruments, prompting a deeper consideration of their role in laboratory settings.
Introduction
Silver electrodes have emerged as essential instruments in pharmaceutical laboratories, where precision and reliability are paramount. Their unique properties, including enhanced stability and superior conductivity, render them invaluable for accurate measurements that can significantly influence research outcomes. As the demand for efficiency and eco-friendliness grows, a critical question arises: how do these metallic conductors balance performance with sustainability? This article explores ten compelling benefits of silver electrodes, illuminating their critical role in advancing pharmaceutical research and laboratory practices.
JM Science Silver Electrodes: Precision for Laboratory Applications
JM Science's silver electrode conductors are meticulously engineered to deliver exceptional precision across various laboratory applications. Their robust design guarantees accurate measurements, which are vital in environments where even minor variations can lead to significant consequences. These sensors are crafted to meet the stringent standards of analytical methods, making them indispensable tools for researchers and technicians alike.
Recent advancements in silver electrode technology, including enhanced conductivity and durability, have further improved their performance, ensuring that medical laboratories can rely on silver electrodes for consistent and trustworthy results. Experts in the field assert that accurate measurements are foundational to successful pharmaceutical research.
Dr. Jane Smith, a leading laboratory researcher, states, "The accuracy of our measurements directly influences the credibility of our research results, making high-quality components crucial for our work." This underscores the critical role that JM Science's silver electrode components play in advancing scientific exploration and enhancing research outcomes, reflecting the company's unwavering commitment to quality and customer support.
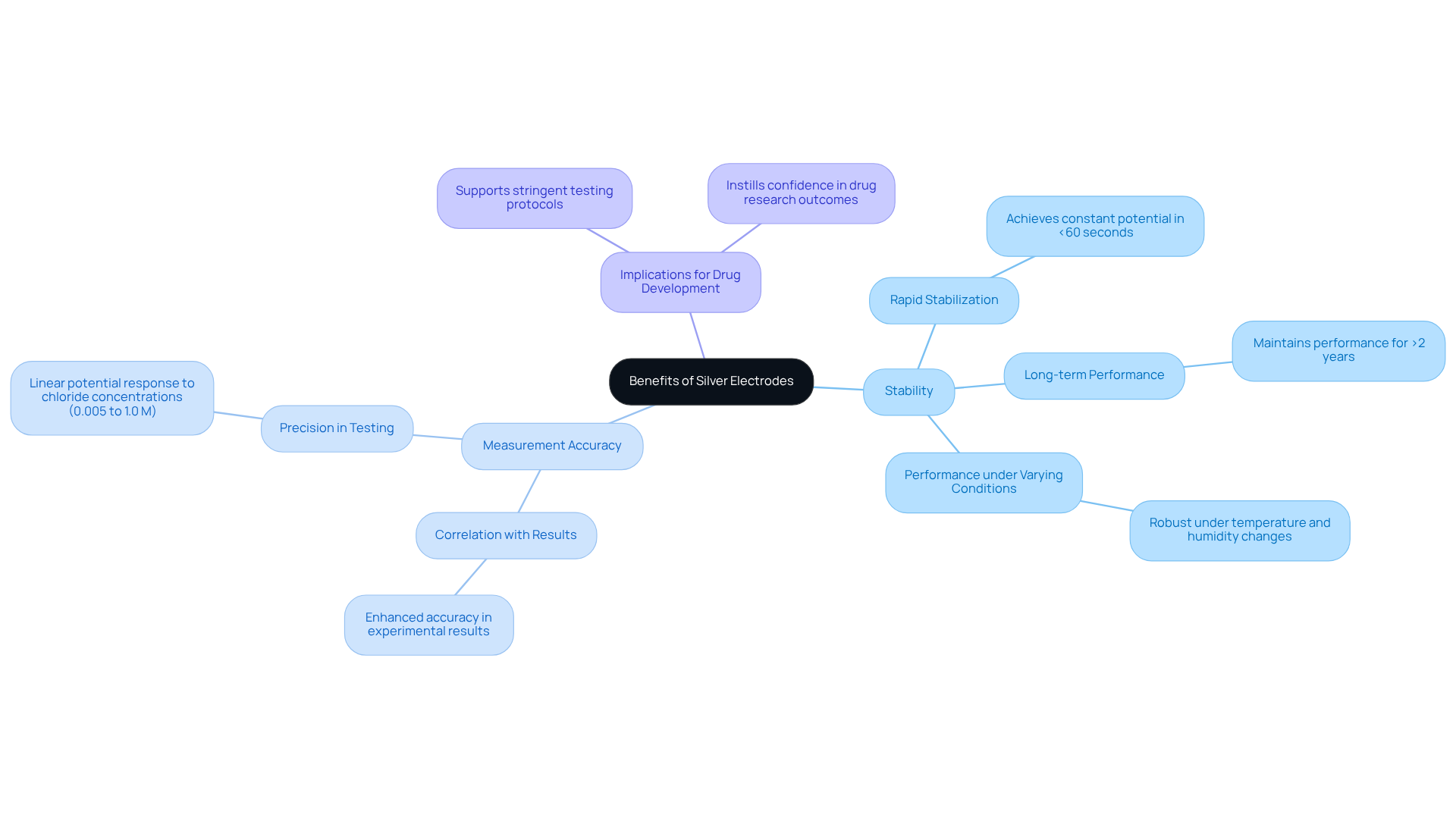
Enhanced Stability: The Key Benefit of Silver Electrodes
Silver electrodes demonstrate remarkable stability, which is a crucial factor in minimizing variations in measurements. This consistency is vital within pharmaceutical laboratories, where precision is paramount for validating research findings. Studies indicate that Ag/AgCl sensors can achieve a constant potential in under 60 seconds—this rapid stabilization is essential for maintaining measurement accuracy in dynamic environments. Moreover, the stability of these components has been demonstrated to endure over extended periods, with certain configurations maintaining performance for more than two years under ambient conditions.
Pharmaceutical lab managers have noted that enhanced stability in silver electrodes directly correlates with improved accuracy in experimental results. The ability of Ag/AgCl sensors to provide a linear potential response to chloride concentrations ranging from 0.005 to 1.0 M facilitates precise tracking of critical parameters in drug formulation and quality control processes. This reliability not only supports stringent testing protocols but also instills confidence in the outcomes of drug research.
Additionally, the benefits of employing metallic conductors extend beyond mere stability. Their robust performance under varying conditions, such as temperature and humidity, guarantees that pharmaceutical labs can achieve consistent results, ultimately leading to more reliable data and informed decision-making in drug development and testing. As the industry increasingly prioritizes accuracy and reproducibility, the significance of stable silver electrodes becomes ever more pronounced.
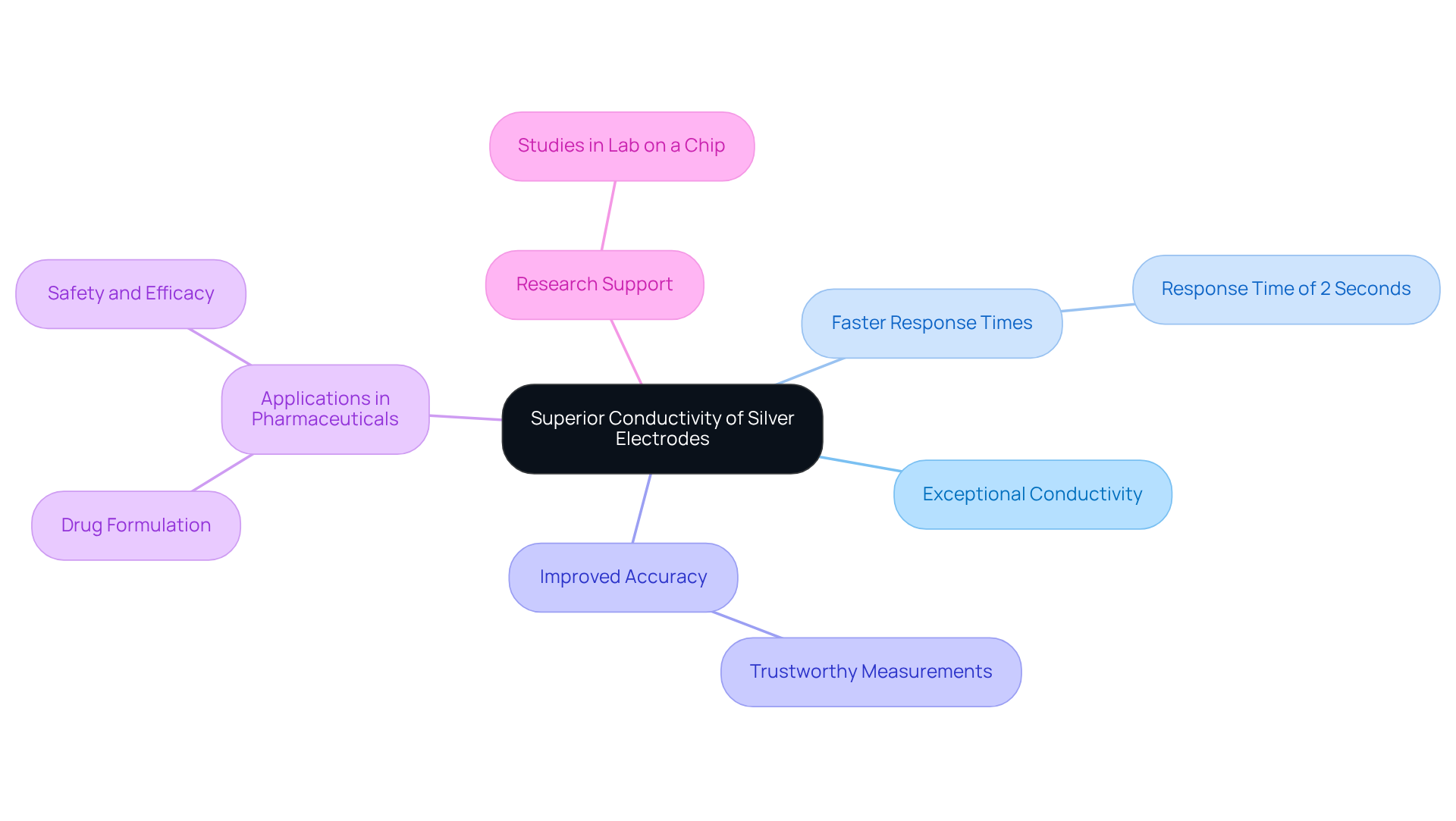
Superior Conductivity: Why Silver Electrodes Excel
Silver electrodes are known for their exceptional conductivity, which significantly enhances their effectiveness in electrochemical applications. This superior conductivity results in faster response times and improved accuracy in readings, rendering them particularly well-suited for a variety of pharmaceutical analyses.
For instance, research indicates that metallic conductors can achieve response times as brief as 2 seconds, facilitating rapid evaluations of low-concentration samples—an essential aspect in drug formulation and testing. Chemists have noted that the ability of metallic conductors to accurately measure minute quantities of materials is vital, especially in scenarios where precision is paramount.
As one chemist asserted, "The high conductivity of metallic conductors enables trustworthy measurements even at low concentrations, which is vital for ensuring the safety and efficacy of medical products."
The significance of conductor conductivity, particularly that of the silver electrode, in medication formulation testing is profound, as it directly impacts the reliability of results, ultimately contributing to the development of safer and more effective medicinal products.
Recent studies published in the journal Lab on a Chip have further substantiated these findings, highlighting the critical role of metallic contacts in enhancing analytical capabilities in drug research.
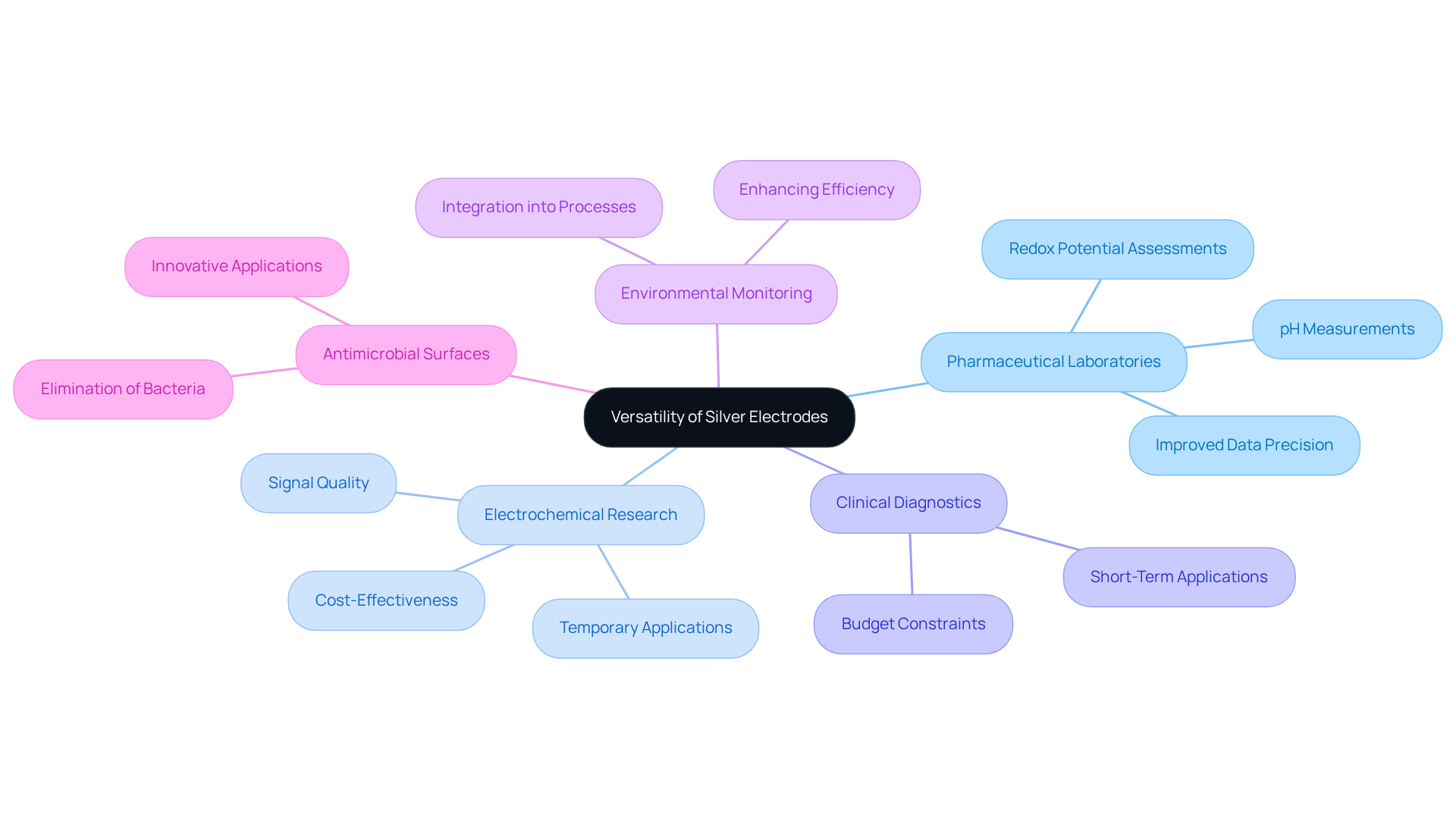
Versatility in Applications: Silver Electrodes Across Disciplines
Silver electrodes serve as versatile instruments in pharmaceutical laboratories, finding applications in a multitude of analytical procedures. The adaptability of the silver electrode is showcased in their use, which spans from pH measurements to redox potential assessments in various experiments. In electrochemical research, metallic conductors are preferred for their affordability and dependable functioning, particularly in temporary applications where financial limitations are a factor. However, it is essential to note that while they initially offer excellent signal quality, oxidation can diminish their performance over time, necessitating more frequent upkeep compared to gold conductors.
The adaptability of these conductive materials transcends mere functionality; they seamlessly integrate into existing laboratory processes, enhancing overall efficiency. Laboratory experts have observed that the inclusion of metallic conductors in experiments not only improves procedures but also increases data precision. Their applications are diverse, spanning clinical diagnostics to environmental monitoring, rendering them indispensable across various scientific disciplines.
Moreover, metallic conductors are increasingly recognized for their role in pioneering studies, including the development of antimicrobial surfaces that leverage their properties to eliminate bacteria. Researchers at the University of Arkansas have created a new antimicrobial surface utilizing nanowires and electric current, underscoring their potential to enhance laboratory practices and improve safety standards. As research continues to advance, the flexibility of conductive materials positions them as essential components in the toolkit of pharmaceutical laboratories, facilitating a broad range of applications and contributing to the progression of scientific understanding.
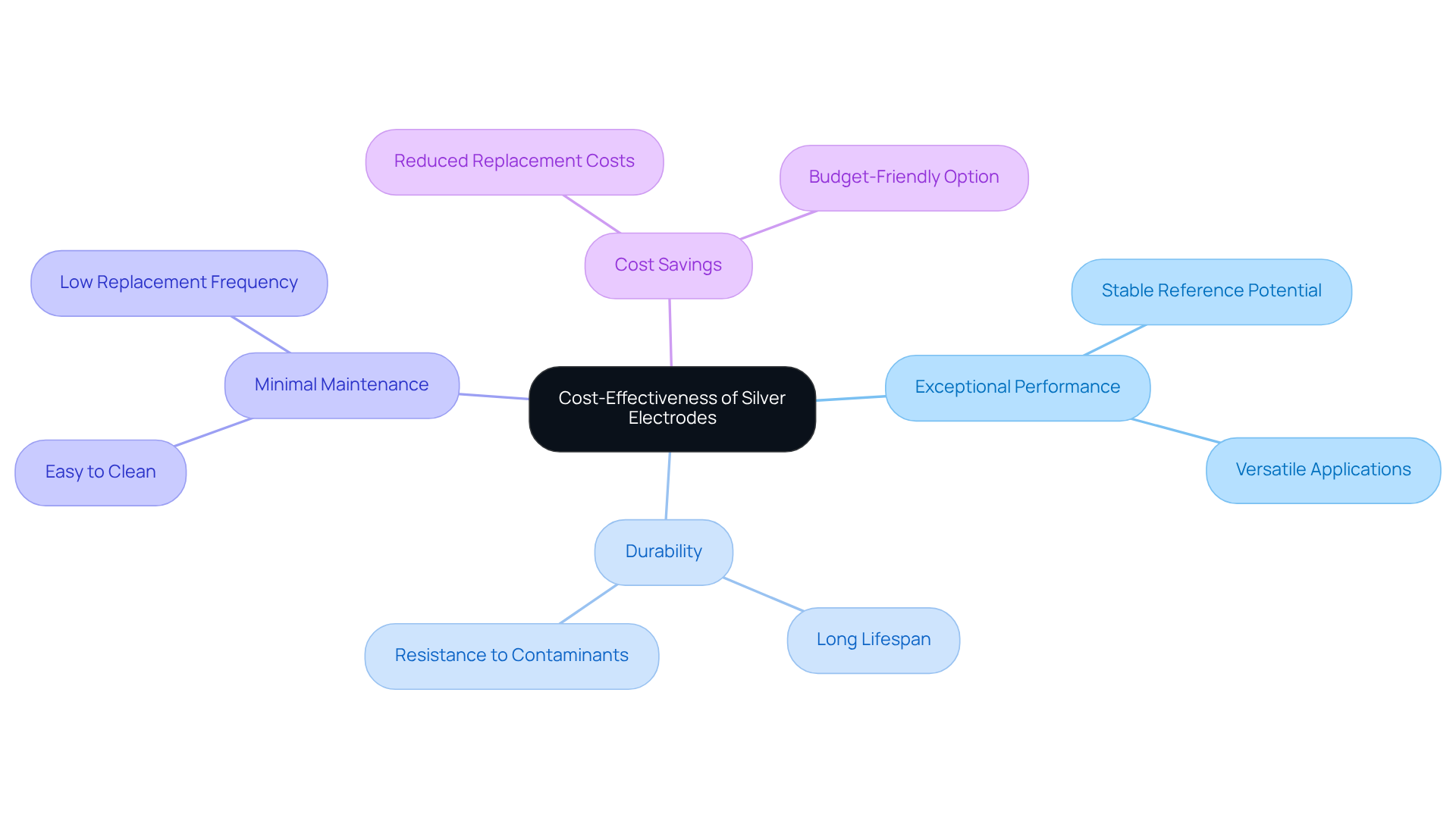
Cost-Effectiveness: Silver Electrodes as a Budget-Friendly Option
Despite their exceptional performance, metallic conductors often present a more economical option compared to other specialized conductors. Their durability and minimal maintenance requirements suggest that research facilities can significantly reduce replacement costs over time. This budget-friendly characteristic positions silver electrodes as an attractive choice for laboratories looking to optimize their resources without compromising on quality. By investing in these high-quality conductors, laboratories can enhance their operational efficiency while ensuring reliability in their scientific endeavors.
Easy Integration: Simplifying Laboratory Setups with Silver Electrodes
Silver electrodes are designed for seamless integration into a variety of experimental configurations. Their broad compatibility with numerous analytical instruments enables laboratories to adopt them swiftly, without the need for extensive modifications. This simplicity not only streamlines the setup process but also facilitates immediate data collection—an essential factor in the fast-paced realm of pharmaceuticals. By incorporating these connectors, labs can enhance their operational efficiency and responsiveness, ultimately leading to improved research outcomes.
Low Maintenance: The Practical Advantage of Silver Electrodes
Silver electrodes offer significant advantages in research environments due to their minimal maintenance requirements. Unlike other variants that often necessitate calibration or replacement, silver electrode sensors are recognized for their durability and consistent performance over extended periods. For example, the AgNP-CF electric field sensor exhibits a potential drift of merely 51.2 µV/24 h, underscoring its reliability. This reliability diminishes the need for constant monitoring, allowing personnel to concentrate on their primary responsibilities, thereby enhancing overall productivity.
A facility supervisor remarked, 'The lifespan of metal conductors has significantly decreased our downtime, enabling us to focus on essential experiments rather than routine maintenance.' Furthermore, the robustness of conductive materials leads to fewer workflow interruptions, a crucial advantage in high-pressure environments such as pharmaceutical laboratories.
Compared to other sensor types, silver electrodes not only reduce maintenance demands but also guarantee consistent results, making them a preferred choice for research facilities aiming to boost efficiency and reliability. Additionally, JM Science offers extensive support materials, including instructional videos and application databases, which further enhance the functionality and dependability of conductive elements in research settings.
Environmental Benefits: Silver Electrodes and Sustainability
Silver conductors are pivotal in promoting sustainability within research environments due to their exceptional durability and recyclability. Their sturdy construction reduces the need for frequent replacements, which significantly curtails waste generation. Additionally, silver is a highly recyclable resource, empowering facilities to adopt more eco-friendly practices.
As Annie Leonard, a staunch advocate for sustainability, asserts, "There is no such thing as ‘away’. When we throw anything away it must go somewhere," this underscores the critical role of recycling in mitigating ecological impact. By opting for silver electrode metallic conductors, laboratories not only enhance their operational efficiency but also contribute to a sustainable future, aligning with the growing emphasis on eco-friendly practices in scientific research.
Furthermore, Pam Shoemaker highlights that recycling is the prudent choice when considering the overall situation, reinforcing the significance of metal components in research sustainability. JM Science Inc. supports this initiative by providing high-quality metal conductors, enabling research facilities to make informed choices that align with their sustainability goals.
Pharmaceutical lab managers are urged to evaluate their current materials and contemplate the integration of silver electrodes to enhance their sustainability initiatives.
Reliability in Critical Experiments: Trusting Silver Electrodes
In critical experiments, the reliability of measurement tools is paramount. Silver electrodes have consistently demonstrated their capability to deliver dependable outcomes, establishing themselves as a trusted choice for drug research facilities. Their performance in high-stakes environments empowers researchers to confidently rely on their data, a necessity for successful drug development and testing.
As industry experts emphasize, consistent results are crucial in drug development, directly influencing the efficacy and safety assessments of new therapies. The significance of measurement reliability on drug development success cannot be overstated; it serves as the foundation for informed decision-making and regulatory compliance.
Silver electrodes exemplify this reliability, providing pharmaceutical labs with the accuracy essential for enhancing their research and ensuring the integrity of their findings.
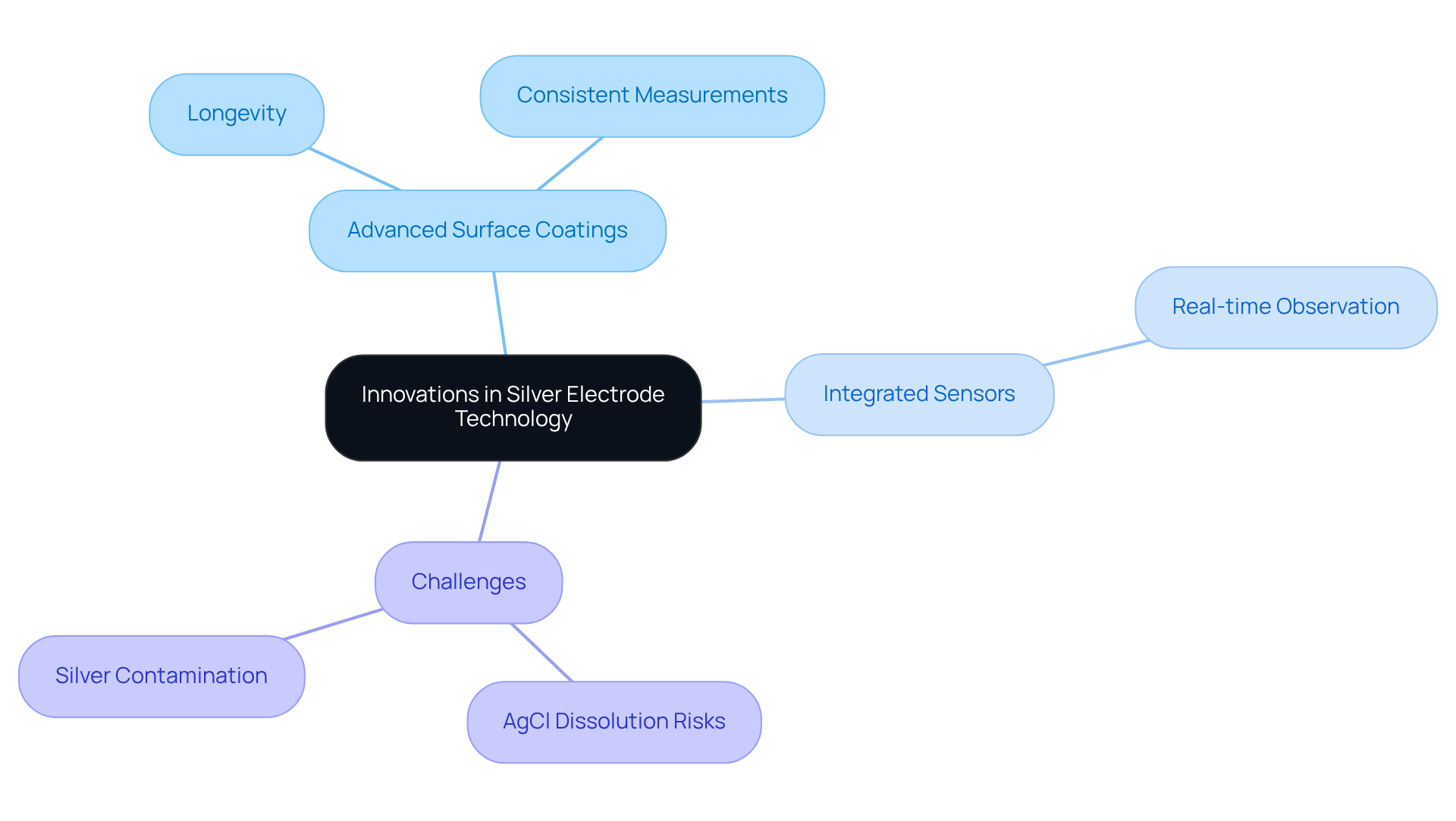
Innovative Features: Advancements in Silver Electrode Technology
Recent advancements in technology related to silver electrodes have significantly enhanced their functionality and usability in laboratory settings. Innovations such as advanced surface coatings and integrated sensors have been developed to improve performance and accuracy. These new coatings not only extend the longevity of the components but also facilitate more consistent measurements under varying experimental conditions. Furthermore, the incorporation of sensors allows for real-time observation of conditions, which is essential for preserving the integrity of delicate experiments.
As noted by Carli Goodfellow, "Known for its exceptional electrical conductivity, this metal plays a crucial role in the efficiency of photovoltaic (PV) cells," highlighting the broader significance of silver in technology. Additionally, Chuhongxu Chen warns that "greater care is required when utilizing Ag/AgCl devices in microfluidic, and especially nanofluidic, systems because AgCl dissolution should not be overlooked," emphasizing the importance of these advancements in addressing potential challenges.
These improvements ensure that metallic conductors remain at the forefront of laboratory technology, equipping pharmaceutical labs with the precise instruments necessary for optimal results. The evolution of silver electrodes highlights a broader trend towards increased efficiency and reliability in scientific instrumentation, emphasizing their vital role in advancing research and diagnostics.
Conclusion
Silver electrodes have emerged as essential components in pharmaceutical laboratories, offering unparalleled precision, stability, and versatility. Their robust design and advanced technology ensure that researchers can rely on accurate measurements, which are critical for the success of drug development and testing. By integrating silver electrodes into laboratory setups, facilities can enhance their operational efficiency and maintain the integrity of their research outcomes.
The article highlights numerous benefits, including:
- Superior conductivity that facilitates rapid response times
- Low maintenance requirements that reduce downtime
- Cost-effectiveness that allows laboratories to optimize their resources
Additionally, the environmental advantages of silver electrodes, such as durability and recyclability, contribute to sustainable practices in research settings. These attributes collectively reinforce the importance of adopting high-quality silver electrodes for reliable and efficient laboratory operations.
Ultimately, pharmaceutical labs are encouraged to evaluate their current measurement tools and consider the integration of silver electrodes to enhance both their research capabilities and sustainability efforts. Embracing these innovative components not only supports scientific exploration but also aligns with the growing demand for accuracy and environmental responsibility in laboratory practices.




