Overview
This article delineates ten pivotal benefits of UPLC columns for pharmaceutical laboratories, underscoring their essential role in enhancing precision, efficiency, and regulatory compliance within analytical processes. By detailing how UPLC technology significantly improves separation efficiency, reduces analysis times, and minimizes solvent usage, it aligns with stringent industry standards. Ultimately, this leads to more reliable results in drug development and quality assurance, reinforcing the necessity of high-quality scientific instruments in laboratory settings.
Introduction
The realm of pharmaceutical laboratories is increasingly defined by the precision and efficiency of analytical tools, with UPLC columns emerging as a game-changer in this landscape. These advanced columns not only enhance separation efficiency and reduce analysis times but also ensure compliance with stringent regulatory standards, making them indispensable for drug development and quality assurance.
As laboratories strive to optimize their workflows and maintain high data integrity, a critical question arises: how can the integration of UPLC technology transform laboratory practices while addressing the challenges of accuracy and cost-effectiveness?
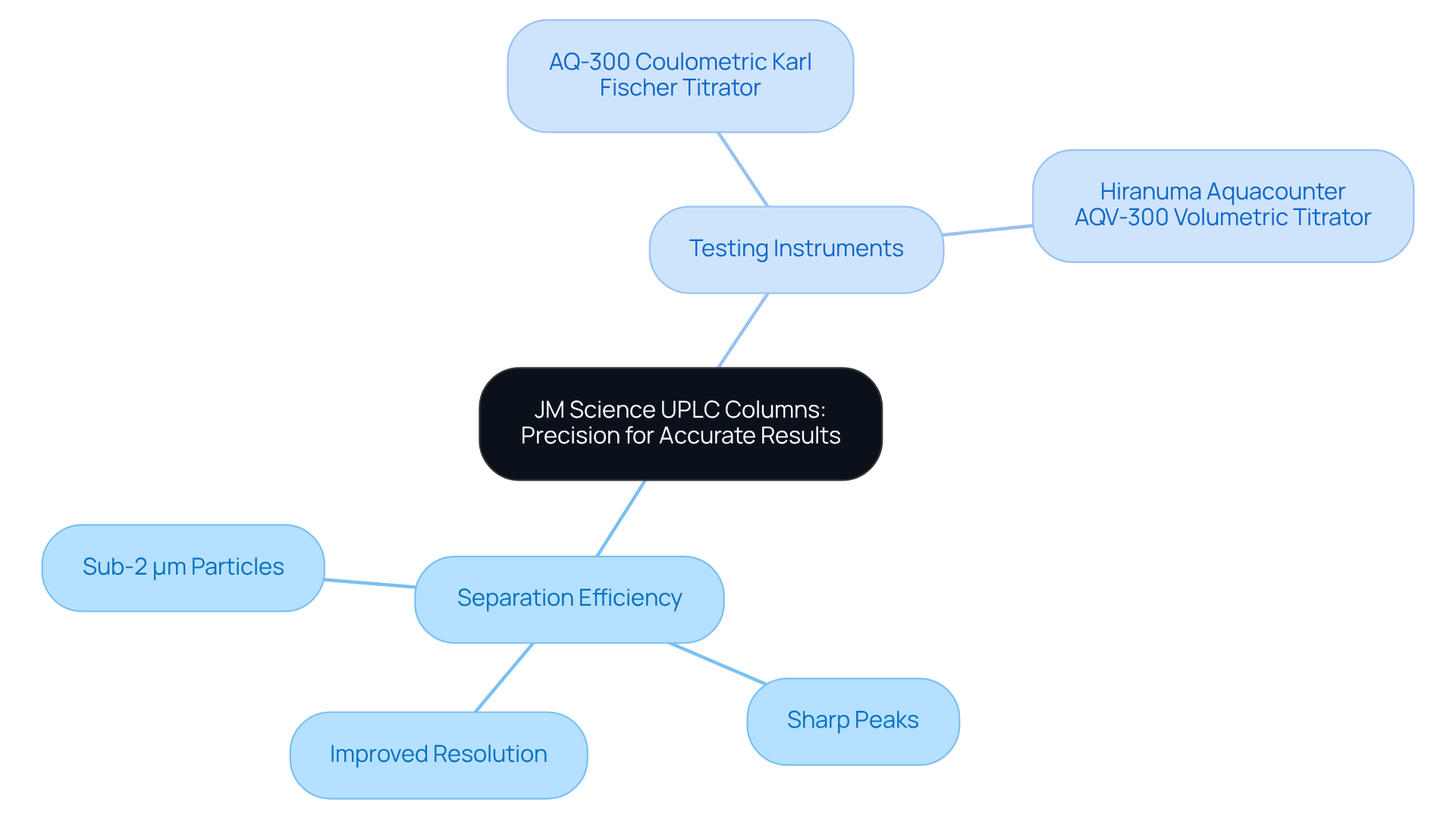
JM Science UPLC Columns: Precision for Accurate Results
JM Science's UPLC column devices stand at the forefront of precision, which is a critical element for achieving accurate results in pharmaceutical environments. The integration of sub-2 μm particles in an uplc column significantly enhances the separation efficiency, yielding sharper peaks and improved resolution. Operating at pressures around 15,000 psi, UHPLC systems that utilize an uplc column facilitate higher efficiencies and expedited separations, making them indispensable in pharmaceutical applications where even minor variations can profoundly impact drug efficacy and patient safety.
Furthermore, the following instruments serve as vital tools for drug and medicine testing:
- AQ-300 Coulometric Karl Fischer Titrator
- Hiranuma Aquacounter AQV-300 Volumetric Titrator
These instruments ensure adherence to the Japanese Pharmacopoeia. By utilizing these advanced devices and titration solutions, laboratories consistently produce reliable results that meet stringent regulatory standards, thereby reinforcing their commitment to quality and compliance in the highly regulated pharmaceutical sector.
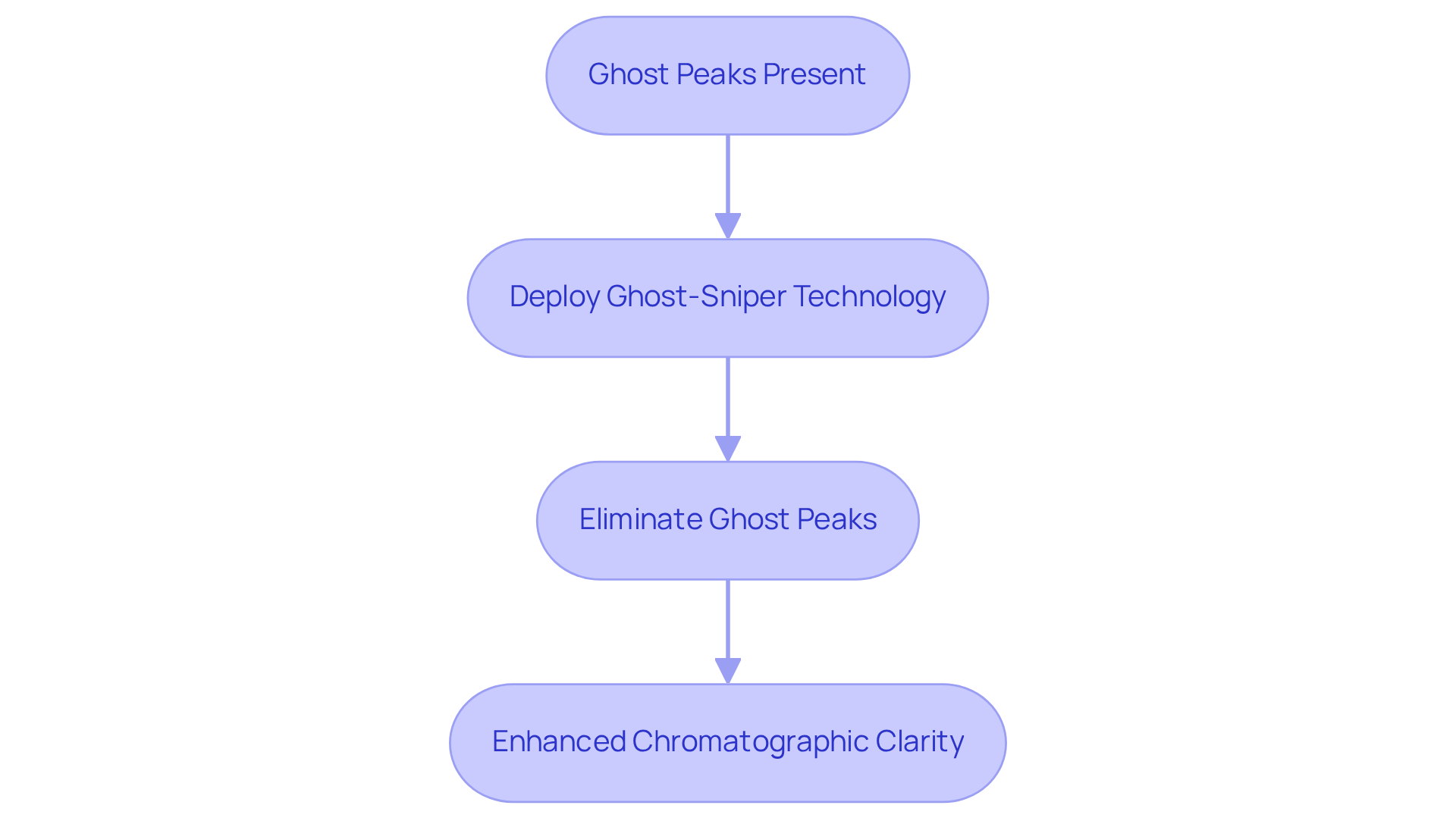
Ghost-Sniper Column: Eliminate Ghost Peaks for Enhanced Clarity
The Ghost-Sniper Column signifies a groundbreaking advancement in chromatography, specifically designed to eliminate ghost peaks that can compromise analytical integrity. Ghost peaks are particularly prevalent in gradient elution methods, leading to misleading interpretations of sample purity and concentration. By effectively capturing these unwanted signals before they reach the detector, this system significantly enhances chromatographic clarity. Analysts have observed that ghost peaks obscure true analyte signals and can result in misidentification of peaks and inaccurate quantification of impurities, ultimately impacting drug development and quality assurance processes.
The deployment of Ghost-Sniper technology empowers research facilities to achieve greater confidence in their data, fostering more reliable results in critical pharmaceutical analyses. Case studies illustrate that the utilization of Ghost-Sniper technology enhances chromatographic clarity, facilitating more accurate identification and quantification of target compounds. Regular substitution of the Ghost-Sniper unit is recommended to maintain its efficiency, ensuring that research facilities uphold high standards in pharmaceutical studies and regulatory compliance.
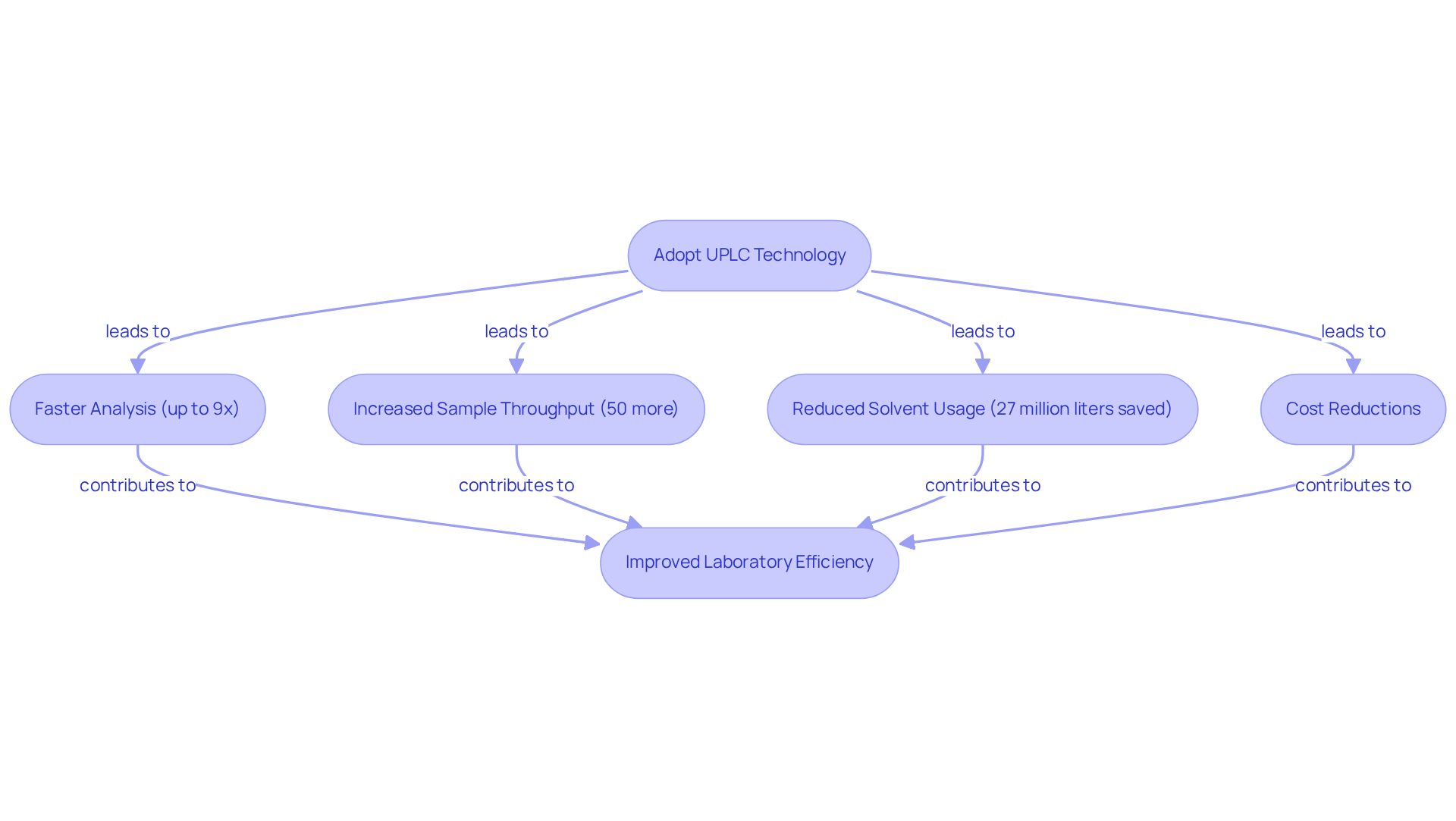
Increased Efficiency: UPLC Columns Streamline Laboratory Workflows
Chromatography columns dramatically enhance efficiency in laboratory environments by significantly reducing analysis durations and increasing throughput. The uplc column technology can perform analyses up to nine times faster than conventional HPLC systems, enabling laboratories to process a greater number of samples within a shorter timeframe. This acceleration not only expedites research timelines—Waters has saved over 300 million hours of operational runtime—but also leads to considerable cost reductions through lower solvent usage, with Waters conserving 27 million liters of mobile phase by utilizing its uplc column, which also results in reduced wear on instruments.
For instance, in pharmaceutical quality assurance, the uplc column used in ultra-performance liquid chromatography has shown an increase in sample throughput by over 50% during paracetamol batch testing, facilitating faster batch releases while maintaining data integrity and adherence to Good Manufacturing Practices (GMP). Furthermore, this technology requires reduced amounts of organic solvents, contributing to more sustainable practices in research settings.
By integrating advanced analytical tools into their workflows, pharmaceutical laboratories can enhance efficiency and concentrate on essential research tasks. Lab managers should consider evaluating their existing systems and exploring advanced liquid chromatography technology, such as uplc columns, to foster innovation and efficiency in their operations.
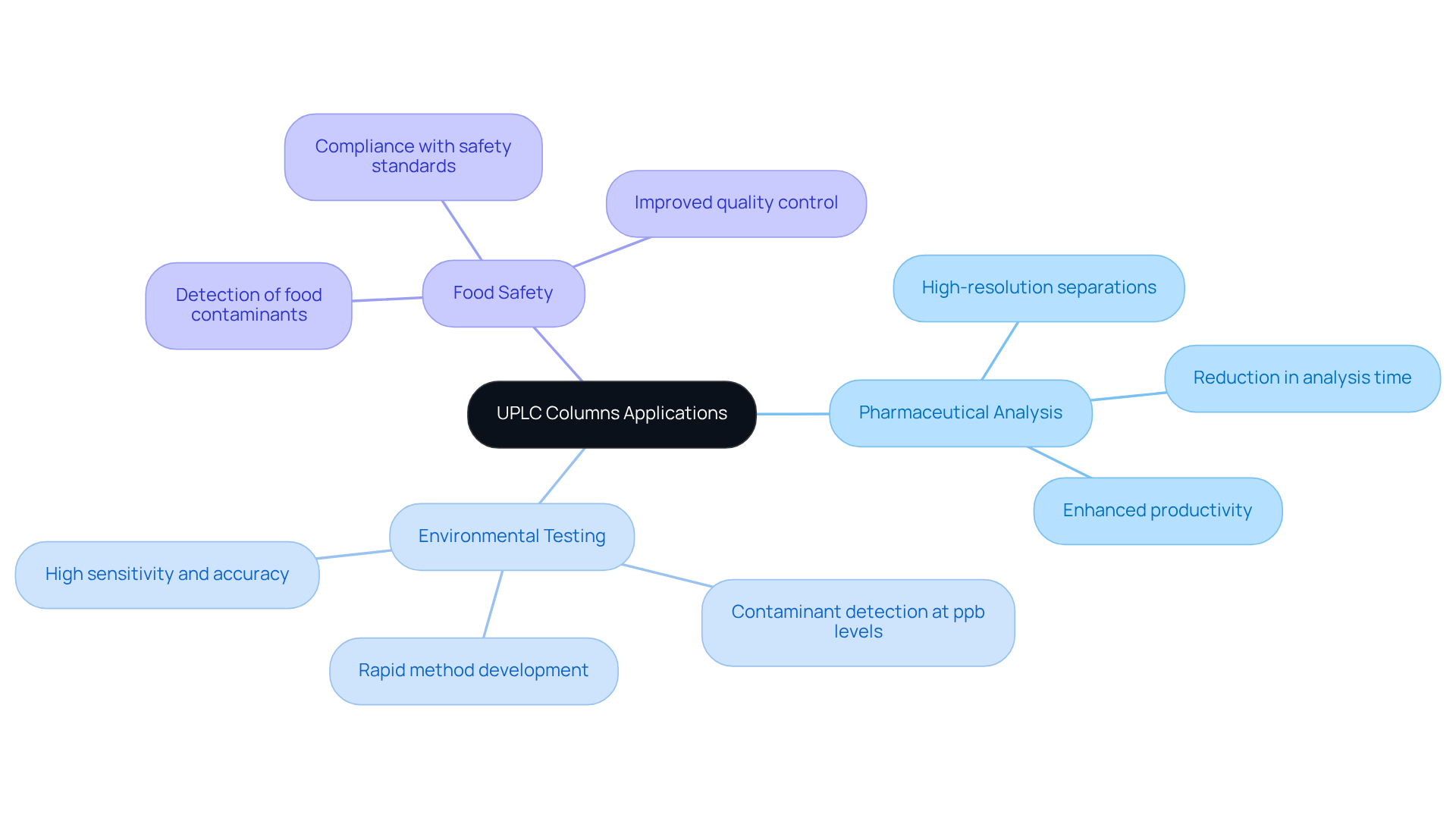
Versatile Applications: UPLC Columns for Diverse Laboratory Needs
The versatility of the uplc column establishes it as an essential tool across various applications, including:
- Pharmaceutical analysis
- Environmental testing
- Food safety
Their capacity to manage intricate combinations while delivering high-resolution separations with an uplc column enables facilities to adapt efficiently to diverse testing requirements. This adaptability proves particularly advantageous in pharmaceutical environments, where varying compounds demand tailored analytical strategies. Notably, uplc columns can achieve a fivefold reduction in analysis time compared to conventional methods, significantly enhancing laboratory efficiency.
Furthermore, their application in food safety is critical, as this technology can detect contaminants at parts per billion levels, ensuring compliance with stringent safety standards. Experts emphasize that the flexibility of this technology, especially when using an uplc column, facilitates seamless integration into existing workflows, making it a preferred choice for facilities aiming to maintain high precision and reliability across a range of analytical challenges.
Cost-Effectiveness: UPLC Columns Deliver Value for Laboratories
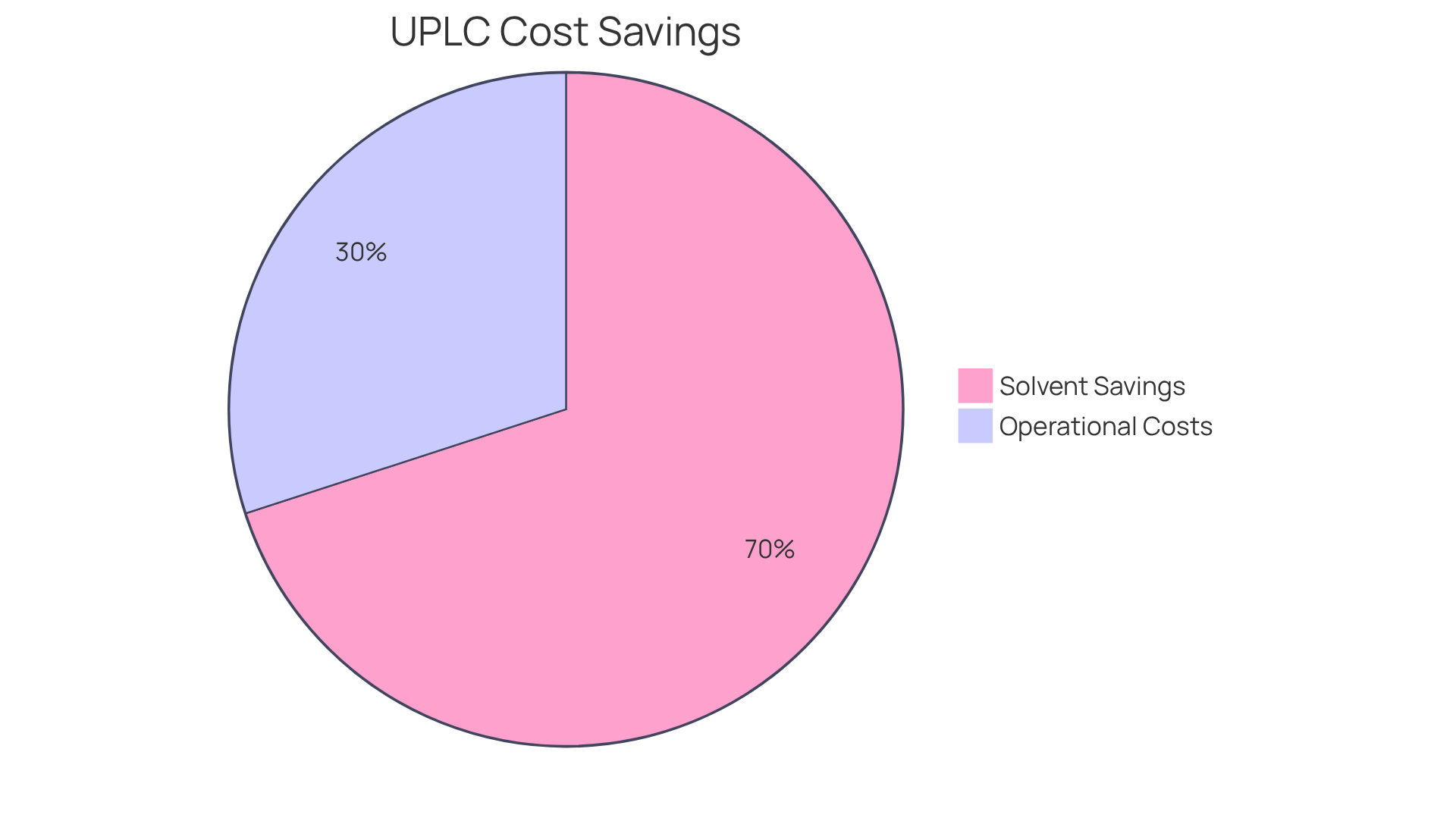
The uplc column provides a highly economical solution for laboratories, primarily through significant reductions in solvent usage and analysis durations. Unlike conventional HPLC techniques, this advanced method can achieve solvent savings of 60-80%, leading to considerable cost reductions over time. For example, a study conducted in pharmaceutical labs revealed that transitioning to advanced liquid chromatography technology reduced solvent usage by approximately 70%, facilitating more efficient resource allocation.
Furthermore, the expedited analysis durations associated with the uplc column enable facilities to enhance sample throughput without requiring additional resources. Financial analysts have observed that these savings can result in a decrease in operational costs by up to 30% annually. This dual advantage of diminished operational expenses and improved efficiency positions these analytical instruments as a strategic investment for laboratories aiming to optimize their workflows while adhering to stringent quality standards.
Moreover, the capability of this technology to analyze complex biological samples underscores its importance in pharmaceutical applications. The integration of these advanced systems not only supports operational efficiency but also enhances the overall effectiveness of laboratory processes.
Technological Advancements: UPLC Columns Enhance Analytical Capabilities
Recent advancements in high-performance liquid chromatography technology, especially involving the uplc column, have significantly enhanced testing capabilities. Innovations such as sub-2 μm particle sizes and improved designs of the uplc column facilitate higher resolution and sensitivity in separations. These developments empower facilities to analyze intricate samples with increased precision and speed, establishing this technology as an essential tool in modern chemical analysis. By adopting the latest analytical technologies, laboratories can ensure they remain at the forefront of analytical performance, meeting the rigorous demands of pharmaceutical testing and research.
![]()
Regulatory Compliance: UPLC Columns Meet Industry Standards
These analytical tools are meticulously designed to adhere to stringent regulatory standards, including Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP). Founded in the 1970s, GLP ensures the quality and integrity of non-clinical research studies, while GMP, enforced by the FDA, focuses on the manufacturing processes to guarantee product safety and quality.
The outstanding performance and dependability of uplc column equipment make them ideal for application in regulated settings, where ensuring data integrity and reproducibility is crucial. By utilizing an uplc column, facilities can conduct analyses that meet regulatory standards, thus enabling more seamless audits and inspections. This adherence to compliance not only protects the facility's reputation but also plays a crucial role in ensuring the safety and efficacy of pharmaceutical products.
As RVJ Niti Samani states, "GMP ensures that drugs are manufactured safely and consistently, whereas GLP ensures that the data collected during the drug development process is of the highest grade." Regulatory specialists stress that the incorporation of advanced chromatography technology, particularly the uplc column, is essential for laboratories seeking to maintain the highest standards of quality and reliability in their operations.
Moreover, failure to adhere to GLP and GMP may result in serious repercussions, such as loss of customer confidence and regulatory fines, highlighting the significance of using advanced chromatography tools to ensure compliance.
Data Quality Improvement: UPLC Columns Ensure Reliable Results
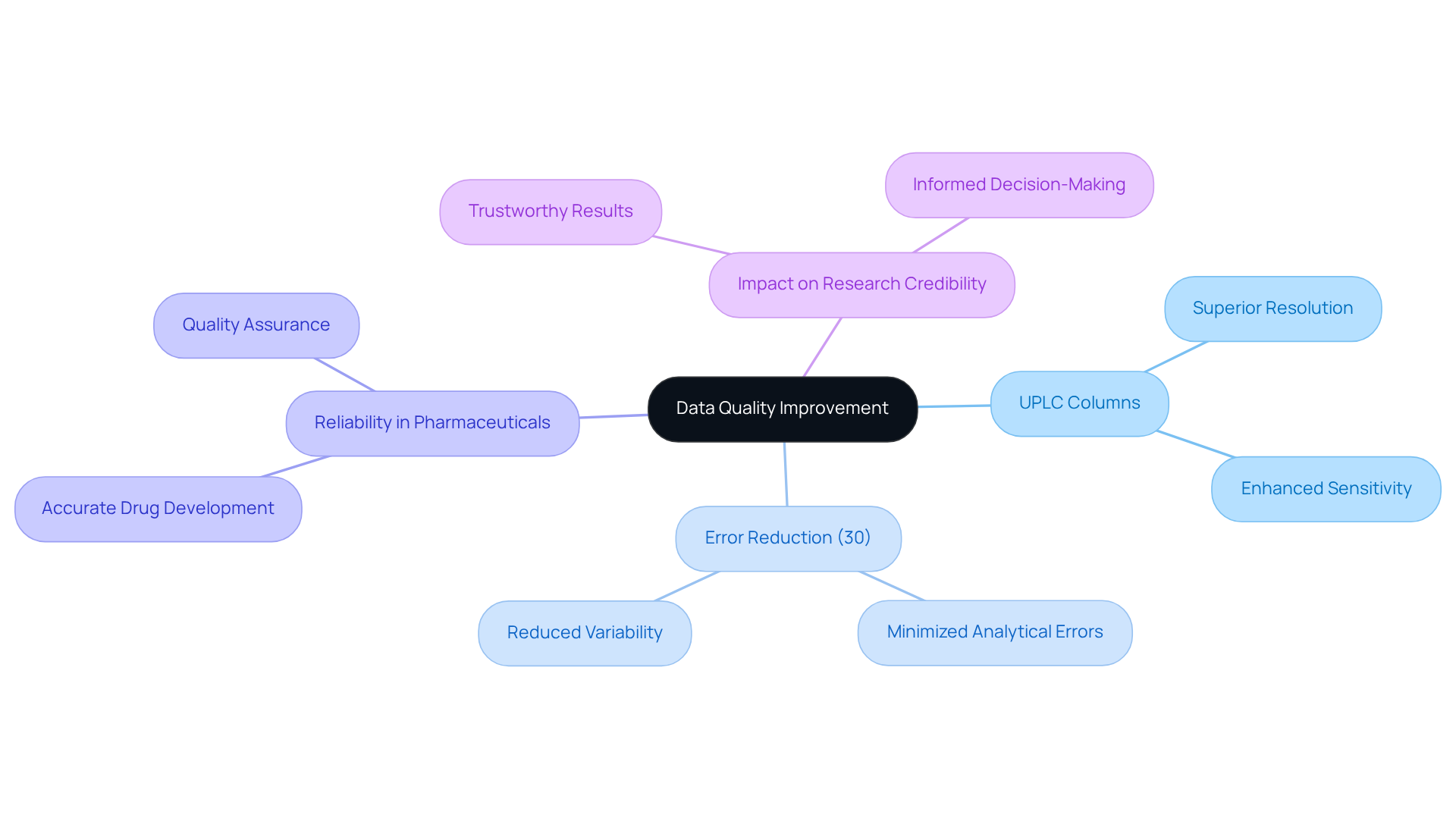
UPLC columns are pivotal in enhancing data quality, ensuring consistent and reliable results. The superior resolution and sensitivity of the uplc column technology significantly minimize errors and variability in analytical data. This reliability is particularly vital in pharmaceutical environments, where accurate data obtained from the uplc column is essential for drug development and quality assurance.
Research indicates that ultra-performance liquid chromatography can achieve error reduction rates of up to 30% compared to conventional techniques, thereby reinforcing the reliability of analytical processes. By ensuring high data quality, these analytical tools bolster the credibility of research findings, enabling scientists to make informed decisions based on trustworthy results.
As industry experts emphasize, the capacity to produce reliable results is not merely advantageous; it is indispensable for advancing pharmaceutical research and safeguarding patient safety.
User Support: Resources for Maximizing UPLC Column Performance
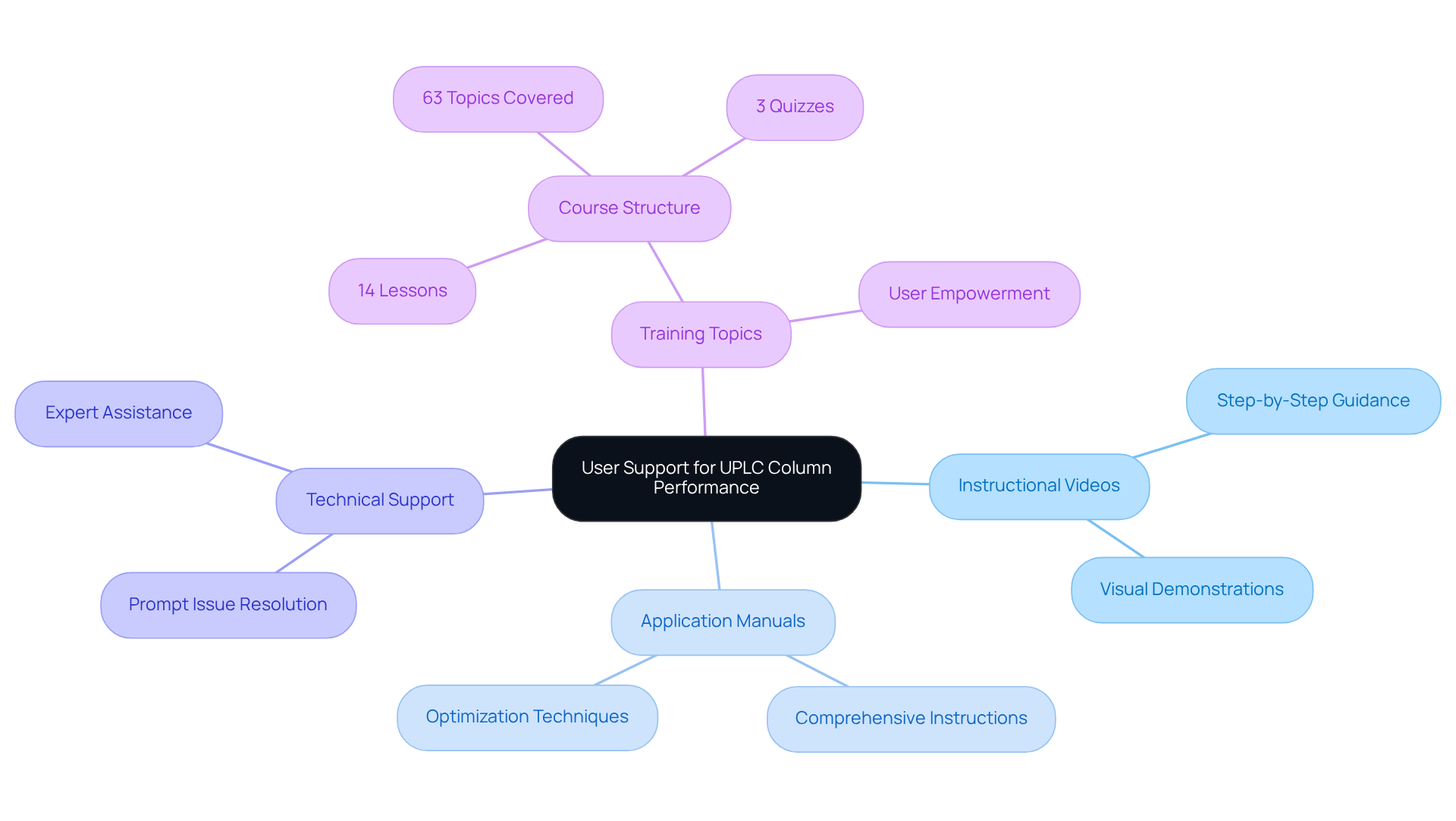
JM Science is dedicated to empowering facilities to fully harness the capabilities of their uplc column and chromatography columns, offering an extensive range of user support resources. This includes a comprehensive collection of instructional videos and application manuals that provide step-by-step guidance for optimizing liquid chromatography technology. With the system's ability to operate at elevated pressures—up to 100M Pa—compared to conventional HPLC, facilities can achieve enhanced testing results and operational efficiency. Additionally, the company provides robust technical support to promptly resolve any challenges encountered during operation. These resources equip staff with the essential skills for effectively utilizing the uplc column, which leads to improved evaluation results and increased operational efficiency.
With 88 enrolled participants and 63 topics covered in their training resources, JM Science ensures clients have access to a wealth of knowledge. This proactive approach to user support underscores JM Science's commitment to fostering successful laboratory practices, enabling clients to achieve the highest standards in their testing endeavors. By prioritizing comprehensive training and support, JM Science not only enhances operational capabilities but also cultivates a culture of excellence within laboratory environments.
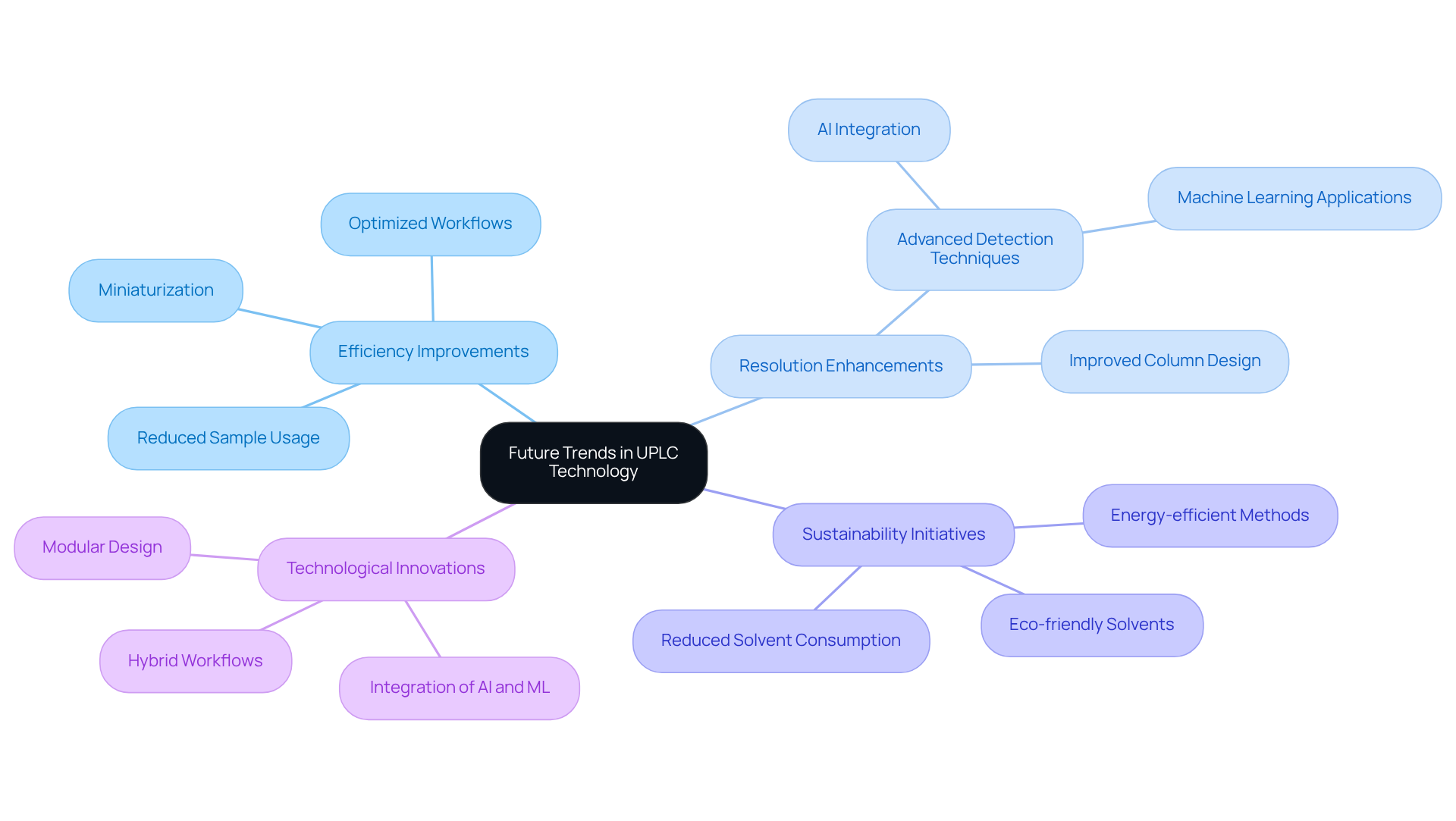
Future Trends: Evolving UPLC Technology for Modern Laboratories
The future of the UPLC column technology is poised for remarkable advancements, driven by ongoing research focused on enhancing efficiency, resolution, and sustainability. Innovations such as the miniaturization of the UPLC column allow for reduced sample and solvent usage, while the integration of advanced detection techniques—particularly through AI and machine learning—significantly improves measurement accuracy. Additionally, the development of eco-friendly solvents addresses the growing demand for sustainable practices in research.
As research facilities embrace these trends, they will be well-equipped to meet evolving evaluation needs and maintain a competitive edge in the pharmaceutical sector. By adopting these advancements, laboratories can enhance their analytical capabilities through the use of an UPLC column, paving the way for breakthroughs in research and healthcare.
Moreover, tackling challenges like economic volatility and regulatory changes will be essential for laboratories to effectively implement these innovations.
Conclusion
The benefits of UPLC columns in pharmaceutical laboratories are multifaceted, encompassing enhanced precision, efficiency, and compliance. By leveraging advanced technology, including the integration of sub-2 μm particles and innovative designs, UPLC columns significantly improve separation efficiency, reduce analysis times, and bolster data quality. These attributes are crucial for pharmaceutical applications, where accuracy and reliability are paramount for drug development and patient safety.
Key insights from the article highlight how UPLC columns, such as the specialized Ghost-Sniper column, effectively address common challenges in chromatography, including ghost peaks that can obscure analytical clarity. The reduction in solvent usage and operational costs further underscores the cost-effectiveness of adopting UPLC technology in laboratory settings. Moreover, UPLC columns serve as versatile tools applicable across diverse fields, including environmental testing and food safety, showcasing their adaptability to various analytical needs.
As laboratories continue to evolve, embracing the latest advancements in UPLC technology is essential for maintaining high standards of efficiency and compliance. Investing in these analytical tools not only enhances operational capabilities but also ensures adherence to regulatory requirements, ultimately safeguarding the integrity of pharmaceutical research. Laboratories are encouraged to assess their current methodologies and consider the integration of UPLC columns to optimize workflows and contribute to advancements in healthcare.




