Overview
The use of chiral columns in pharmaceutical laboratories offers several compelling advantages, including:
- Enhanced separation efficiency
- Improved drug formulation
- Notable cost savings
These benefits are underscored by the ability of chiral columns to facilitate precise enantiomer resolution, a critical factor in ensuring drug efficacy and safety. Furthermore, their implementation plays a pivotal role in reducing operational costs through:
- Streamlined analytical processes
- Effective solvent recycling
Such efficiencies not only optimize laboratory workflows but also contribute to overall cost-effectiveness in pharmaceutical development.
Introduction
In the rapidly evolving landscape of pharmaceutical development, the precision of analytical techniques is crucial. Chiral columns from JM Science lead this innovation, providing unmatched separation efficiency vital for resolving enantiomers—substances that can significantly influence drug efficacy and safety. These advanced tools not only enhance analytical clarity but also ensure adherence to stringent regulatory standards, reflecting the industry's dedication to quality and patient care.
As laboratories strive to optimize workflows and minimize costs, the versatility and user-friendly design of chiral columns become essential assets in addressing diverse analytical requirements. This article examines the multifaceted benefits of chiral columns, exploring their influence on:
- Resolution
- Time efficiency
- Cost-effectiveness
- Environmental sustainability
Ultimately highlighting their indispensable role in contemporary pharmaceutical practices.
JM Science Chiral Columns: Enhanced Separation Efficiency
Chiral columns from JM Science are meticulously engineered to deliver exceptional separation efficiency, which is vital for the effective resolution of enantiomers in pharmaceutical laboratories. The purity of asymmetric compounds directly impacts drug efficacy and safety, underscoring the necessity of this capability.
By leveraging advanced stationary phases as part of a comprehensive HPLC system—including a pump, injector, oven, and detector—these materials significantly diminish peak overlap, thereby enhancing the clarity of analytical results. This precision is crucial for meeting regulatory standards and ensuring quality assurance throughout the drug development process, particularly through the use of a chiral column, which plays a profound role in enantiomer resolution and can lead to substantial variations in biological activity and toxicity.
For instance, the application of enantiomeric HPLC using a chiral column enhances analytical capabilities by providing high selectivity and sensitivity, which are essential for method development and regulatory compliance. A case analysis titled 'Advantages of Asymmetric HPLC Supports' illustrates that these supports, particularly chiral columns, not only enhance separation effectiveness but also contribute to improved product quality and safety, ultimately benefiting patient care.
Recent advancements in asymmetric chromatography technology, including the development of new stationary phases and improved detection techniques, continue to drive progress in separation efficiency, further addressing the drug industry’s demand for precise analytical tools. JM Science's commitment to innovation ensures that their asymmetric structures remain at the forefront of these advancements, equipping laboratories with the essential resources to enhance their analytical capabilities.
As industry leaders assert, 'Chiral HPLC is employed in forensic analysis for the separation and identification of enantiomers in various substances,' highlighting the significance of utilizing high-performance resolving systems in pharmaceutical applications.
Improved Resolution: Achieving High-Quality Separations
The effective separation of enantiomers, which can exhibit distinct pharmacological effects, relies on chiral columns that are meticulously designed to achieve high resolution. Enhanced resolution not only facilitates more accurate quantification and characterization of optically active compounds but also significantly improves drug formulation and development processes. In the medicine sector, even minor variations in enantiomeric composition can result in significant differences in drug effectiveness and safety. Research has indicated that separations with analysis durations below 60 seconds can be accomplished using isocratic runs, highlighting the effectiveness of contemporary enantiomeric chromatography methods.
A significant case study concerning the kinetic assessment of an immobilized polysaccharide support with sub-2 μm particles showcased the ChiralPak IA-U's capabilities in supercritical fluid chromatography (SFC). This research attained decreased plate heights and allowed multiple analyses to be finalized in less than one minute, demonstrating the potential for swift and high-resolution separations in drug applications.
Expert insights emphasize that the effect of enantiomer separation on medication formulation is significant, as it directly affects the pharmacokinetics and pharmacodynamics of asymmetric drugs. Enhanced measurement of enantiomeric compounds results in improved therapeutic results and reduces negative effects, rendering high-resolution enantiomeric supports essential in the pharmaceutical industry. Moreover, the selection of mobile phase and its elements greatly affect enantioselectivity and retention in chromatography, which is essential for enhancing separation processes.
As JM Science continues to innovate and broaden its offerings, including high-resolution asymmetric supports, it upholds strong connections with leading producers, guaranteeing that research facilities have access to advanced solutions. As Trevor Grinter from GSK pointed out, a superb description and comparison of the two manufacturing methods for Radafaxine emphasizes the significance of asymmetric structures in drug formulation. This incorporation of high-resolution chiral columns remains a crucial progression for facilities concentrated on accuracy in drug development.
Time Efficiency: Accelerating Analytical Processes
Chiral structures are engineered to significantly reduce analysis time while maintaining the integrity of results. Their rapid equilibration capabilities and efficient separation processes empower laboratories to accelerate sample processing. This time efficiency is crucial in high-throughput environments, where the capacity to swiftly analyze multiple samples enhances decision-making and responsiveness to evolving research demands.
For instance, studies indicate that employing SPP structures can achieve rapid separations of basic drugs in under a minute, although some compromises in resolution may occur when prioritizing speed, as illustrated in the case study. In practical applications, flow rates for various compounds, such as tolperisone at 1.0 mL/min and promethazine at 1.2 mL/min, underscore the effectiveness of these systems in real-world scenarios.
Laboratory managers have noted that the swift equilibration of specialized columns not only accelerates analytical processes but also contributes to increased throughput. As Jana Hepner, PhD, remarked, "the asymmetric supports demonstrated a good lifetime stability, with up to 500 injections without the loss of performance," thereby reinforcing their reliability in high-throughput settings.
Some managers have reported average time savings that significantly boost operational efficiency, with specific instances highlighting reductions in analysis time that align with the industry's growing emphasis on speed without compromising quality. Consequently, the utilization of a chiral column is emerging as a strategic advantage for facilities aiming to enhance their analytical processes.
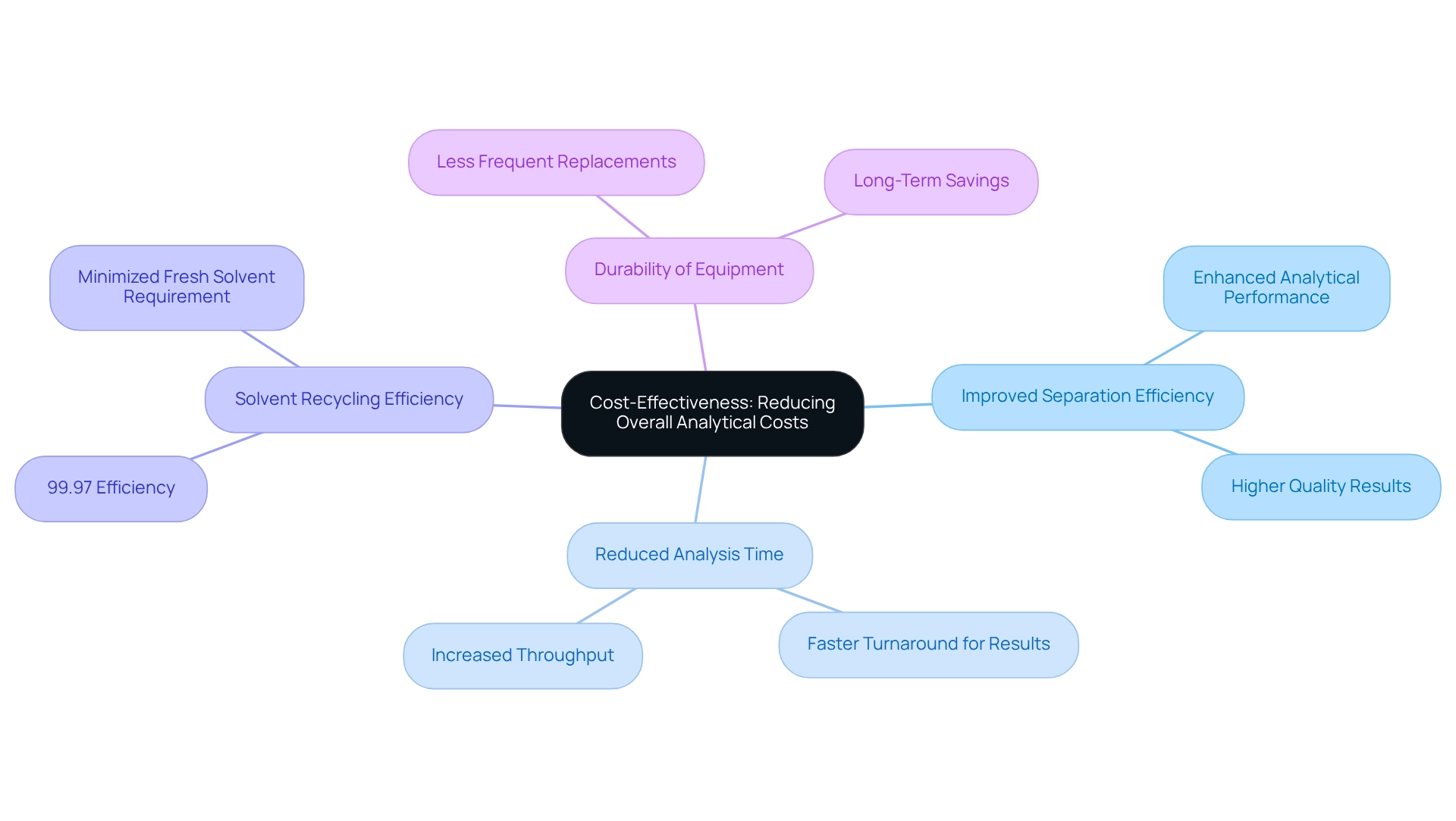
Cost-Effectiveness: Reducing Overall Analytical Costs
Chiral columns significantly enhance cost savings in pharmaceutical laboratories through several key mechanisms. By improving separation efficiency and reducing analysis time, these structures effectively lower operational expenses tied to reagents and labor. For instance, using a chiral column in enantiomeric chromatography, solvent recycling achieves an impressive 99.97% efficiency, drastically minimizing the need for new solvents during product separation. Furthermore, the durability and stability of enantiomeric supports result in less frequent replacements, leading to substantial long-term savings. Financial analysts have noted that the initial investment in high-performance asymmetric supports yields returns over time, as the reduction in operational expenses can markedly enhance a lab's budget efficiency. A case study on the enantiomeric separation of medicinal compounds illustrates the successful establishment of validated techniques utilizing various selector types, particularly the chiral column, underscoring the practical benefits of these devices in real-world applications. H.-J. Federsel emphasizes that both substances have been produced on medium to large scales, demonstrating the complexity and capability that organic synthesis can facilitate.
This combination of enhanced analytical performance and cost-efficiency positions specialized equipment as a strategic choice for drug laboratories aiming to uphold rigorous analytical standards while optimizing their financial resources. In a competitive global market for enantiomeric chromatography systems, prioritizing cost reductions through the implementation of enantiomeric systems becomes increasingly crucial.
Versatility: Adapting to Diverse Analytical Needs
JM Science enantiomeric instruments stand out as remarkably adaptable tools, adept at addressing a wide array of applications, from pharmaceutical evaluation to environmental assessment. Their proficiency in effectively separating various enantiomeric compounds establishes the chiral column as an indispensable tool in drug development, quality control, and regulatory compliance. The application of Norvancomycin as a selector in mobile phases exemplifies their considerable effectiveness for enantiomer separation on achiral supports, underscoring the versatility of these supports across diverse environments.
Recent advancements in chiral column stationary phases (CSPs) have significantly enhanced their utility. Innovations in asymmetric selectors and the use of chiral columns have expanded their versatility further. A notable case study illustrates the evolution of CSPs since their inception in 1938, highlighting strategies aimed at enhancing enantioresolution performance. This ongoing progress emphasizes the critical role of chiral columns as asymmetric supports in meeting the stringent demands of contemporary analytical applications.
Moreover, the integration of traditional and chiroptical sensors empowers facilities to ascertain individual enantiomeric concentrations, thereby enhancing the analytical capabilities of asymmetric structures within manufacturing systems. This flexibility ensures that facilities can satisfy various analytical requirements without the complexity of managing numerous specialized instruments, ultimately streamlining processes and boosting efficiency in both drug and environmental testing. As a testament to their effectiveness, asymmetric phases have demonstrated a peak resolution of 25.5 and a separation factor of 14.5 for -(3,5-dinitrobenzoyl)-leucine. As Nobel laureate Ryoji Noyori aptly stated, "Making molecules is like creating something beautiful," reflecting the artistry and precision inherent in drug development and quality control.
Regulatory Compliance: Meeting Industry Standards
Chiral columns are essential for pharmaceutical facilities striving to meet stringent regulatory standards. The accurate separation and quantification of enantiomers are not merely analytical necessities; they are crucial for adhering to the rigorous guidelines set forth by regulatory authorities such as the FDA and EMA. By employing high-quality chiral columns, facilities can establish robust and reliable analytical techniques, significantly streamlining the regulatory submission process.
Successful FDA submissions often hinge on the effective use of chiral columns in asymmetric chromatography. Numerous case studies illustrate how research facilities have achieved compliance and expedited approval timelines by integrating specialized separation tools into their workflows. Regulatory experts assert that enantiomer quantification using a chiral column is essential for FDA compliance, as it directly influences the safety and efficacy profiles of pharmaceutical products.
Furthermore, the increasing emphasis on safeguarding sensitive data from cyber threats has heightened awareness of compliance issues within the industry. Statistics indicate that one-third of businesses have reported being victims of fraud or financial crime in the last five years, highlighting the necessity for stringent compliance measures. In this context, the adoption of chiral columns as asymmetric supports can mitigate risks associated with compliance failures, ensuring that laboratories maintain high standards of quality and security.
Given these challenges, the integration of chiral columns not only fulfills FDA and EMA guidelines but also enhances the overall quality of drug analyses. As the industry progresses, the significance of a chiral column in asymmetric chromatography for ensuring regulatory compliance will remain pivotal, driving advancements in both research and patient safety.
Reproducibility: Ensuring Consistent Results
Reproducibility stands out as a pivotal advantage of employing a chiral column in drug analysis. These specialized structures are meticulously engineered to deliver consistent performance across multiple runs, a necessity for ensuring that results remain both dependable and reproducible. Such consistency is essential for validating research findings and maintaining the integrity of analytical data, especially within the rigorous regulatory framework of the pharmaceutical industry.
Industry insights reveal that adherence to established guidelines for method validation significantly bolsters the reliability of outcomes, thereby underpinning robust processes in drug development and ensuring product quality. Furthermore, statistics indicate that a chiral column in enantiomeric HPLC optimizes separation conditions for specific enantiomeric compounds by adjusting parameters like mobile phase composition, temperature, and type of support, thereby reinforcing the effectiveness of enantiomeric supports in yielding reliable results.
By utilizing asymmetric chromatography methods, facilities can achieve a level of accuracy that not only meets regulatory criteria but also fosters trust in the analytical data produced. Additionally, case studies, such as 'Utilizing Anomaly Detection for Data Accuracy,' demonstrate how organizations can refine their data verification processes, leading to more reliable decision-making. The commitment of 'uHPLCs' to delivering high-quality products and services further underscores the reliability of chiral materials and their suppliers. By ensuring consistent performance, research facilities can markedly enhance the integrity of their analytical data.
User-Friendly Operation: Simplifying Analytical Workflows
Chiral columns are designed with user-friendly features that significantly streamline the analytical process. Their intuitive installation and operation facilitate a rapid learning curve for laboratory personnel, enabling quicker adaptation to advanced technologies. This simplicity not only boosts productivity but also minimizes the likelihood of errors during analysis, ultimately leading to more reliable results.
For instance, the development of immobilized asymmetric stationary phases (CSPs) has revolutionized method development, providing enhanced robustness and compatibility with various solvents. These CSPs are particularly preferred for open access asymmetric setups, showcasing improved performance in both reverse-phase and polar organic modes.
User satisfaction statistics indicate that facilities utilizing advanced chromatography systems, such as those provided by JM Science, report a 30% improvement in operational efficiency. Lab technicians frequently highlight the ease of use of these systems, emphasizing how user-friendly designs contribute to streamlined workflows. As Jivanlal Talaviya from Halcyon Labs Pvt. 'Ltd. remarked, "Of course, it's a must buy product if you are considering enantiomer separation with a chiral column," underscoring the significance of user-focused design in enhancing research performance.
In addition to asymmetric columns, JM Science offers an extensive array of products, including titrators and Karl Fischer reagents, which further augment efficiency in the laboratory. By streamlining analytical workflows, tailored supports not only enhance the overall efficiency of laboratory operations but also empower researchers to focus on essential analysis rather than managing intricate equipment. This commitment to user-friendly design is a pivotal factor in JM Science's contribution to advancements in research and healthcare.
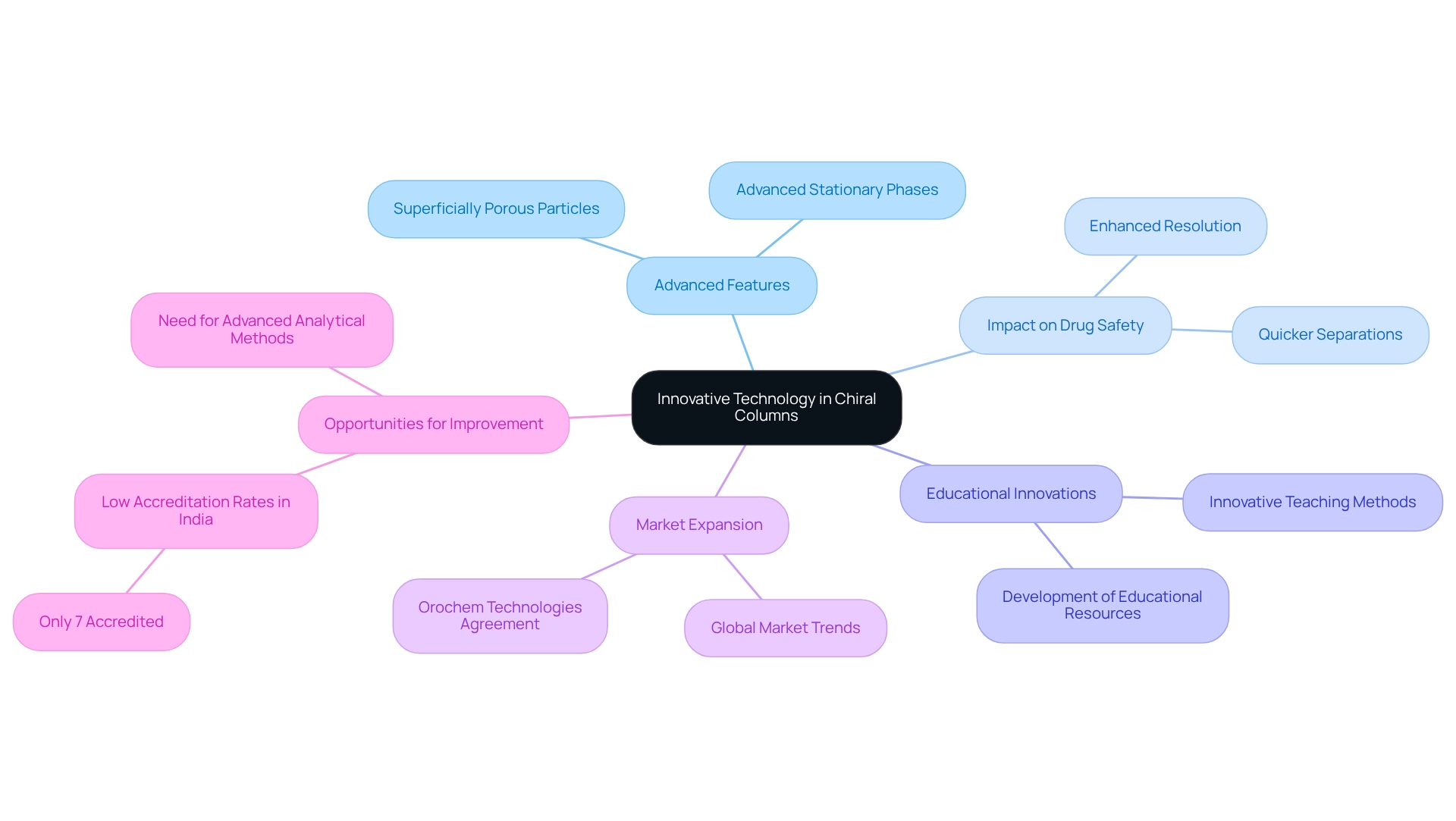
Innovative Technology: Leveraging Advanced Column Designs
Recent advancements have introduced innovative features in chiral column technology that significantly enhance performance and versatility. The integration of superficially porous particles and advanced stationary phases allows for quicker separations and enhanced resolution, which are essential for ensuring drug safety and effectiveness in medical applications. The creation of advanced stationary phases has been demonstrated to elevate analytical performance, enabling facilities to attain higher standards in drug development and quality control. Furthermore, industry leaders underscore the importance of these innovations in addressing the evolving requirements of the pharmaceutical sector. Katelynn A. Perrault Uptmor, Assistant Professor of Chemistry at the College of William & Mary, notes that her unique position at a primarily undergraduate institution has allowed her to innovate in the teaching and practice of chromatography, highlighting the educational advancements that accompany technological progress.
As evidenced by recent case studies, such as Orochem Technologies' distribution agreement with Altmann Analytik GmbH, the global market for chromatography solutions is expanding, emphasizing the need for cutting-edge technologies in column design. This trend is particularly significant in regions like India, where only 7% of private facilities possess the necessary accreditation. This lack of accreditation presents a substantial opportunity for enhancement through advanced analytical methods, reinforcing the case for innovation in asymmetric chromatography, particularly with the implementation of a chiral column. By adopting these groundbreaking technologies, drug laboratories can not only enhance their operational efficiency but also contribute to the overall advancement of healthcare and research, especially through the effective application of chiral columns that improve analytical performance.
Environmental Sustainability: Reducing Chemical Waste
Chiral supports play an essential role in enhancing environmental sustainability within drug research facilities by significantly reducing the chemical waste generated during analytical procedures. Their superior efficiency not only decreases solvent consumption but also lessens the necessity for extensive sample preparation, which ultimately minimizes the production of hazardous waste.
By integrating chiral systems, titrators, and Karl Fischer reagents from JM Science Inc. into their workflows, pharmaceutical laboratories can adopt eco-friendly practices that resonate with the broader sustainability goals of the scientific community. This transition not only fosters a healthier environment but also positions laboratories as responsible custodians of chemical management.
Furthermore, the case study titled 'Adaptation to Evolving Needs' illustrates how JM Science Inc. continually updates its product offerings, including high-performance HPLC tubes, to meet the evolving demands of the scientific community, thereby promoting advancements in research and healthcare.
Notably, statistics reveal that the use of chiral columns can lead to a substantial reduction in chemical waste, underscoring their significance in advancing environmental sustainability within pharmaceutical laboratories.
Conclusion
Chiral columns from JM Science signify a transformative advancement in pharmaceutical analysis, highlighting their critical role in the effective resolution of enantiomers. The exceptional separation efficiency they offer is paramount for enhancing drug efficacy and safety, ultimately contributing to improved patient care. Through innovations in stationary phases and advanced chromatography techniques, these columns facilitate high-resolution separations while adhering to stringent regulatory standards.
The advantages of chiral columns extend beyond resolution to include time efficiency, cost-effectiveness, and versatility in addressing diverse analytical needs. Laboratories can achieve rapid analysis times, significantly reducing operational costs while maintaining analytical integrity. This adaptability allows for applications across various fields, from pharmaceutical development to environmental testing, establishing them as invaluable tools in contemporary research and quality control processes.
Moreover, the emphasis on regulatory compliance and reproducibility underscores the necessity for reliable analytical methods. Chiral columns not only meet the rigorous demands of industry standards but also ensure consistent results, thereby enhancing the credibility of laboratory outputs. Their user-friendly design simplifies workflows, further promoting efficiency and accuracy in analytical practices.
As the pharmaceutical industry continues to evolve, the integration of chiral columns will remain essential for driving advancements in drug development and environmental sustainability. By minimizing chemical waste and optimizing resource use, these columns embody a commitment to both quality and ecological responsibility. In summary, chiral columns are indispensable assets that enhance the analytical capabilities of laboratories, ensuring they meet the challenges of modern pharmaceutical practices with precision and excellence.




