Overview
The article titled "10 Common Errors During Titration and How to Fix Them" serves as an essential resource for identifying frequent mistakes encountered in titration processes and offers solutions aimed at enhancing accuracy. It meticulously outlines various errors, including:
- Endpoint misjudgment
- Volume misreading
- Contamination
To address these issues, the article suggests practical strategies such as:
- Improved training
- Consistent cleaning protocols
- Implementation of automated systems
These measures are crucial for mitigating errors, thereby ensuring more reliable analytical results. This overview underscores the significance of precision in scientific practices, encouraging readers to adopt these recommendations for improved outcomes.
Introduction
Errors during titration can significantly compromise the accuracy of analytical results, presenting challenges that laboratories must navigate with precision. This article explores ten common pitfalls encountered during titration processes, offering effective strategies to rectify them. As the demand for reliable measurements intensifies, it raises an important question: how can laboratories enhance their practices to ensure consistent, high-quality outcomes?
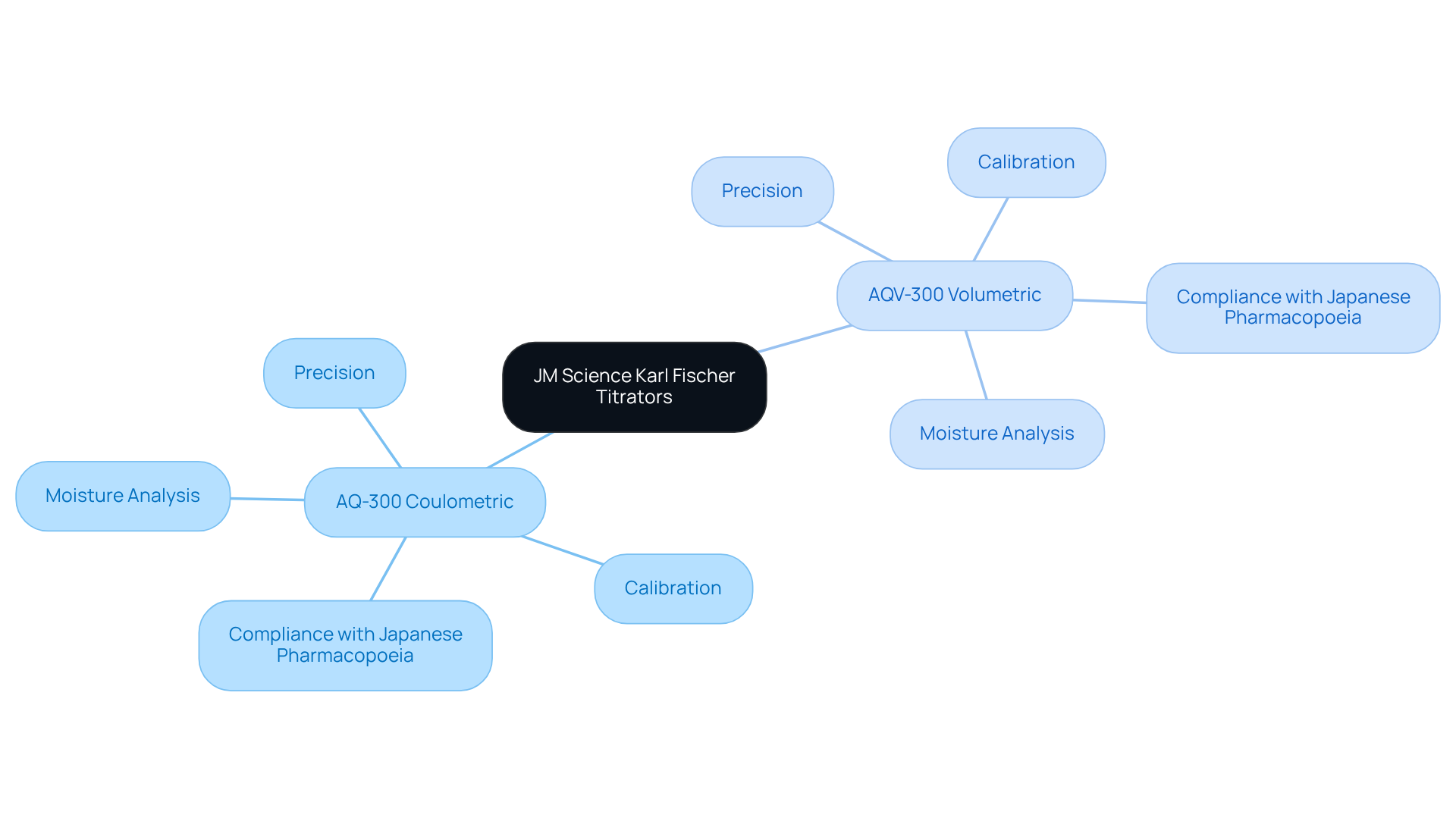
JM Science Karl Fischer Titrators: Precision in Moisture Analysis
JM Science presents advanced Karl Fischer titrators, featuring the AQ-300 Coulometric and AQV-300 Volumetric models, meticulously engineered for precise moisture analysis in pharmaceuticals and other samples. These instruments employ a well-defined electrochemical method to determine water content, thereby ensuring exceptional accuracy and reproducibility in compliance with the Japanese Pharmacopoeia.
Utilizing these titrators enables facilities to significantly reduce errors associated with moisture assessment, a critical factor in the pharmaceutical and chemical sectors. Furthermore, regular calibration and maintenance of these devices enhance their reliability, establishing them as indispensable tools in any analytical environment.
The AQ-300 and AQV-300 titrators are also equipped with features that facilitate suitability testing for drugs and medicines, reinforcing their vital role in upholding product quality.
End Point Error: Recognizing and Correcting Misjudgments
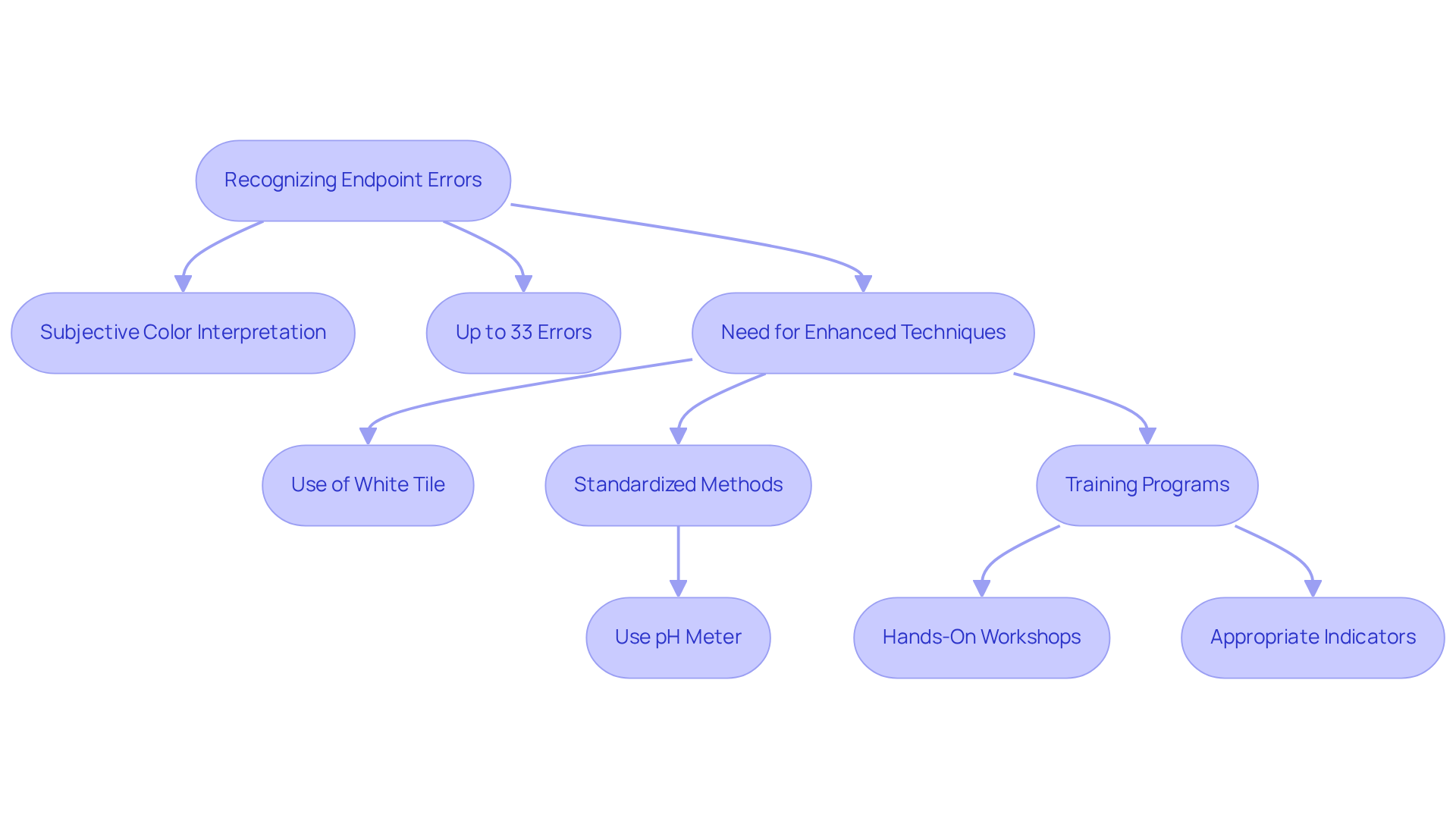
Errors during titration in the process frequently stem from misjudging the endpoint, largely due to subjective interpretations of color changes. Research shows that a considerable percentage of titrations—up to 33%—are influenced by this subjective color perception, which highlights the urgent need to address errors during titration with enhanced techniques.
To address these errors, implementing consistent practices is paramount. Employing a white tile can significantly improve the visibility of color changes, facilitating the detection of subtle shifts. Furthermore, adopting standardized methods for endpoint determination—such as the use of a pH meter or a colorimetric indicator—can substantially minimize errors during titration.
Training programs designed to enhance staff's ability to recognize subtle color changes are vital. Methods such as hands-on workshops, simulations, and the selection of appropriate indicators can effectively reduce endpoint misjudgment. As one lab manager noted, 'Adequate training in identifying color changes is crucial for guaranteeing measurement precision.'
By fostering a deeper understanding of color changes and their implications for measurement accuracy, laboratories can achieve more reliable results. This proactive strategy not only enhances individual performance but also bolsters overall laboratory efficiency and precision.
Volume Misreading: Techniques to Ensure Accurate Measurements
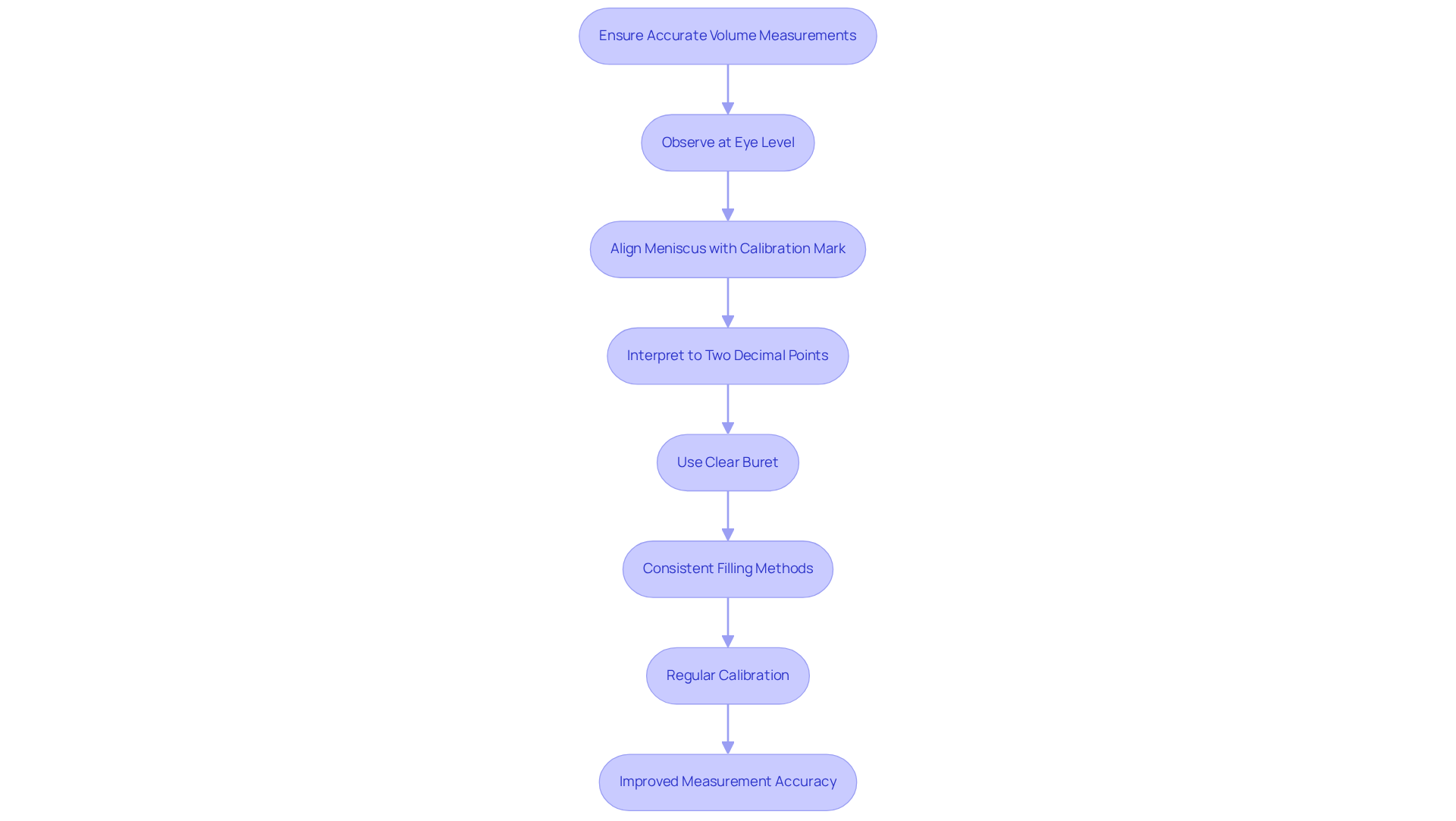
Errors during titration are a prevalent challenge, frequently stemming from improper eye level or incorrect placement of the dispensing apparatus, leading to volume misreading. To achieve accurate measurements, it is imperative to observe the graduated cylinder at eye level, ensuring that the meniscus aligns precisely with the calibration mark. Interpreting the device to two decimal points is essential for enhanced precision. Employing a buret with clear, well-defined markings can significantly reduce errors. Consistent filling methods, such as avoiding overfilling and allowing the liquid to stabilize before taking a reading, further bolster measurement accuracy.
Regular calibration of instruments is equally vital for maintaining precision in measurement outcomes. Inaccurate readings can lead to costly errors and wasted resources, highlighting the necessity of meticulous measurement practices.
Practical examples illustrate the impact of these practices:
- In a pharmaceutical laboratory, technicians adhering to correct eye-level reading methods observed a notable decrease in volume misreadings, resulting in more reliable measurement outcomes.
- Moreover, analytical chemists assert that common volume misreading issues often result in errors during titration, which can be traced back to a lack of attention to these fundamental practices.
As Robert H. Grubbs aptly noted, 'The key to scientific discovery is the ability to recognize and address errors.' By implementing these techniques, laboratories can substantially improve the precision of their measurement processes, ultimately facilitating better decision-making in research and quality control.
Equipment Misuse: Best Practices for Proper Titration Techniques
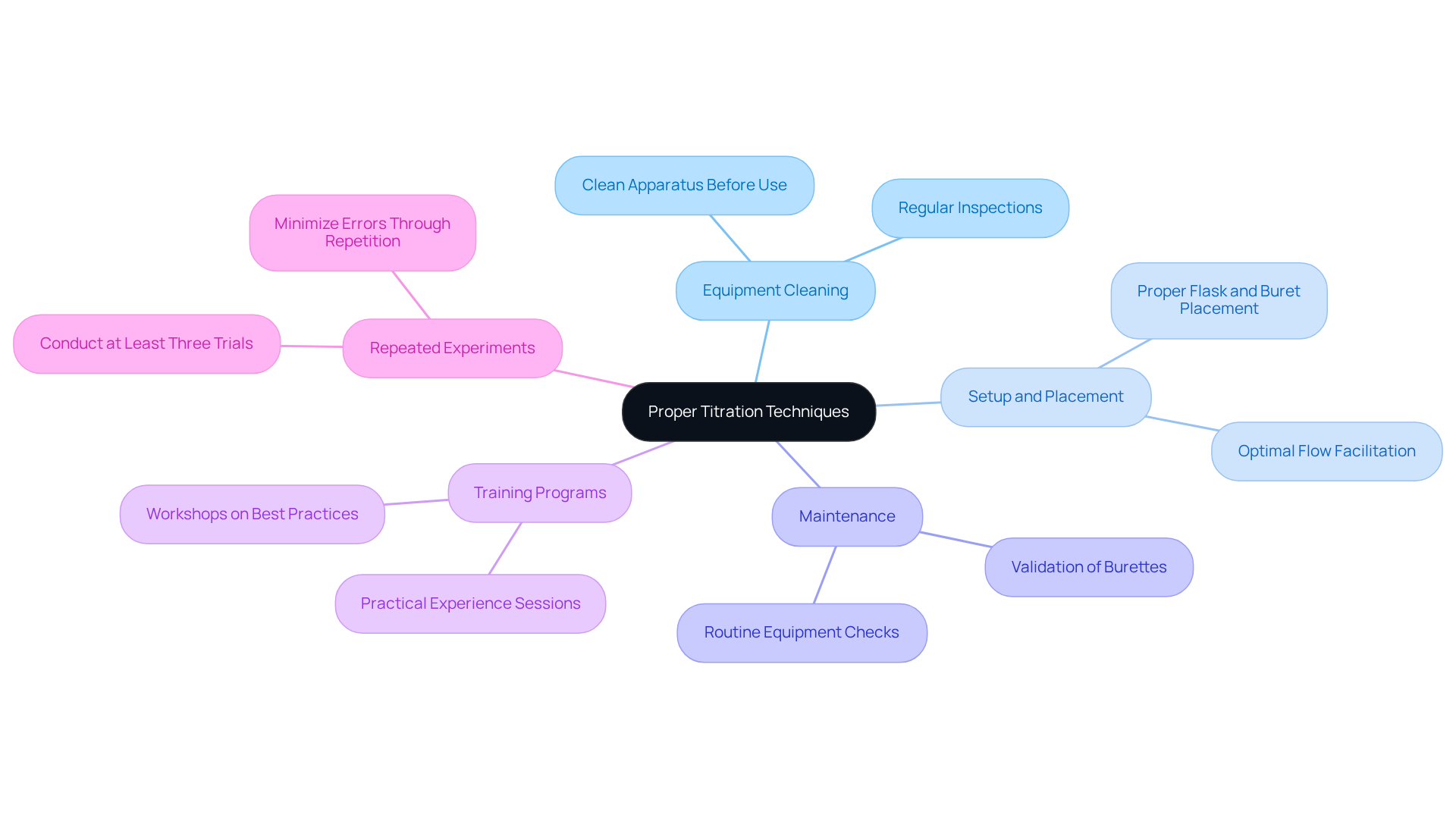
Correct equipment utilization is vital to prevent errors during titration and ensure precise measurement outcomes. To begin, ensure that the apparatus is meticulously cleaned and devoid of any residual chemicals, as even trace amounts can cause errors during titration. Understanding the specific setup for the procedure is essential to prevent errors during titration, which includes the proper placement of the flask and buret to facilitate optimal flow and measurement. Regular inspections and maintenance of equipment are crucial to prevent errors during titration that could compromise accuracy. Research indicates that conducting repeated experiments at least three times per analyte significantly enhances accuracy and helps minimize errors during titration.
Furthermore, implementing training programs focused on equipment handling, such as workshops emphasizing best practices and practical experience, can substantially reduce errors during titration in research environments. As one laboratory instructor aptly stated, "Grasping the subtleties of measurement techniques is essential for dependable results."
Moreover, cleaning burets prior to use is a critical practice that must not be overlooked, as it directly impacts the occurrence of errors during titration and the integrity of the analysis process. Additionally, as Jessica Clifton, Director at ReAgent, noted, "When performed correctly, an experiment involving measurement is very reliable," highlighting the significance of proper techniques and maintenance to prevent errors during titration.
Concentration Errors: Ensuring Correct Reagent Dilutions
Concentration mistakes, or errors during titration, often stem from the improper dilution of reagents, which can significantly affect analysis results. To mitigate these errors, utilizing calibrated volumetric flasks during solution preparation is essential, as they guarantee precision in volume measurement. For instance, the effective implementation of automated dilution procedures, such as the vitamin B1/B6 workflow at Reinier de Graaf Hospital, demonstrates that employing calibrated equipment enhances both precision and reproducibility in reagent preparation.
Furthermore, verifying the concentration of prepared solutions through secondary methods, like titration against a standard solution, is a prudent practice to avoid errors during titration. This approach not only confirms the accuracy of the dilution but also strengthens the reliability of subsequent analytical results. Regular training on dilution techniques is vital for laboratory personnel, fostering a deeper understanding of the critical nature of precision in reagent preparation. As Dr. Frans van der Horst, a Clinical Chemist, aptly noted, "Common concentration errors can lead to significant discrepancies in experimental outcomes," highlighting the necessity for meticulous attention to detail in all dilution processes.
Parallax Error: Strategies to Improve Visual Accuracy
Parallax errors occur when the observer's eye is misaligned with the measurement scale, leading to inaccurate readings. Research shows that errors during titration can account for a significant percentage of inaccuracies in results, with some studies indicating they may contribute to as much as 10% of measurement errors. To address this issue, it is essential to ensure that your eye is level with the meniscus during measurements.
Utilizing a buret equipped with a digital display can effectively eliminate parallax errors, providing precise readings without the risk of misalignment. Furthermore, training technical staff to recognize and correct for parallax errors can significantly enhance measurement accuracy. As one specialist noted, maintaining correct eye alignment is crucial for obtaining dependable outcomes in measurement processes.
Additionally, using appropriately sized burets is vital; for instance:
- 10 mL burets have a tolerance of ±0.02 mL
- 50 mL burets have a tolerance of ±0.05 mL
By implementing these methods, research facilities can improve the overall precision of their analyses.
Contamination: Maintaining Cleanliness to Avoid Titration Errors
Contamination poses a significant threat to the accuracy of titration results, which can lead to errors during titration, making rigorous cleaning protocols essential. To ensure precise measurements, all glassware and equipment must be meticulously cleaned prior to use. Implementing dedicated equipment for specific reagents is crucial in minimizing cross-contamination risks. Many analytical facilities have adopted a two-step cleaning process:
- A thorough wash with detergent followed by rinsing with distilled water.
- A final rinse with the reagent to be used.
Regular audits of cleanliness reinforce the significance of a contamination-free setting and assist in identifying potential areas for enhancement. Quality control supervisors frequently stress that 'a clean lab is a precise lab,' emphasizing how cleanliness is crucial to avoid errors during titration and ensure measurement accuracy. As Milton Hershey aptly stated, 'Give them quality. That’s the best kind of advertising,' emphasizing the importance of upholding high standards in testing practices. Furthermore, W. Edwards Deming noted that 'quality is everyone's responsibility,' which is vital in fostering a culture of cleanliness. By following these protocols, laboratories can significantly improve the dependability of their measurement outcomes and reduce errors during titration.
Air Bubbles in Buret: Prevention Techniques for Accurate Titration
Air bubbles in the measuring device can lead to significant inaccuracies during the process, adversely affecting the precision of results. To address this issue, it is crucial to fill the container slowly, allowing the liquid to flow without introducing air. Ensuring that the tip of the dispensing apparatus is filled with the titrant before commencing the titration is imperative. Softly tapping the container can aid in removing any trapped air bubbles, further enhancing measurement precision. Regular inspections for leaks or defects in the buret are vital for maintaining its integrity and ensuring reliable results.
Statistics indicate that employing bubble prevention techniques can improve accuracy by up to 15%, underscoring the necessity of meticulous filling practices. Laboratory technicians emphasize that even tiny air bubbles can cause significant errors during titration, highlighting the need for careful handling. Techniques such as:
- Pre-filling the container
- Utilizing a funnel
can also contribute to more precise titration measurements. Additionally, periodically calibrating the buret and ensuring it is clean before use are essential practices for maintaining measurement accuracy. By implementing these strategies, laboratories can significantly mitigate the impact of air bubbles, leading to more reliable and consistent outcomes in their analyses.
Visual Perception Errors: Enhancing Color Change Detection
Visual perception errors can significantly impact the detection of subtle color changes during processes. To enhance the accuracy of color change detection, it is essential to conduct experiments in well-lit environments and utilize white backgrounds, as these factors improve contrast. Furthermore, training personnel to recognize specific color changes associated with various indicators is crucial for elevating accuracy.
Employing digital colorimeters offers objective measurements of color changes, thereby minimizing reliance on subjective visual interpretation. By implementing these strategies, laboratories can ensure more reliable outcomes in their analyses.
Automated Titration: Reducing Human Error for Reliable Results
Automated dosing systems, such as those offered by JM Science Inc., play a vital role in minimizing human errors during titration, significantly enhancing the reliability of results. These sophisticated systems meticulously control the addition of titrant, guaranteeing uniform outcomes and boosting throughput. Laboratories embracing automation report a notable reduction in variability, resulting in more precise analyses.
Moreover, many automated systems feature data logging capabilities that improve traceability and ensure adherence to rigorous regulatory standards. Laboratory supervisors have noted that the integration of automation has transformed their workflows; one remarked that it has 'revolutionized our method for measurements, enabling us to concentrate on more intricate analyses while relying on the precision of our automated systems.'
By reducing the likelihood of human errors during titration, automated titration not only elevates the quality of results but also streamlines workflows, positioning it as an essential investment for modern analytical facilities. Additionally, JM Science provides a diverse array of products, including HPLC solutions and Karl Fischer reagents, which further enhance laboratory efficiency and accuracy.
To successfully implement automation, it is recommended that managers conduct a comprehensive assessment of their existing processes to pinpoint areas where automation can yield the greatest advantages.
Conclusion
Errors in titration can significantly compromise the accuracy of analytical results, making it crucial for laboratories to adopt effective strategies for error prevention and correction. Understanding common pitfalls—such as endpoint misjudgment, volume misreading, and contamination—enables laboratories to enhance measurement precision and ensure the reliability of their outcomes.
Key insights reveal that employing consistent practices, including:
- Proper training
- Utilizing calibrated equipment
- Maintaining cleanliness
can drastically reduce errors. Techniques such as:
- Using white tiles for better color contrast
- Ensuring correct eye alignment
- Implementing automated systems
are essential for achieving accurate titration results. Furthermore, regular calibration and maintenance of equipment, alongside strict adherence to cleaning protocols, are vital for sustaining measurement integrity.
Ultimately, fostering a culture of precision and attention to detail within laboratories is imperative. By prioritizing training and implementing best practices, facilities can mitigate errors during titration and enhance the overall quality of their analyses. Embracing these strategies will lead to more reliable results, reinforcing the importance of meticulousness in scientific research and quality control processes.




