Overview
This article highlights the significance of ten essential chromatography detectors employed in pharmaceutical laboratories, underscoring their critical roles in facilitating precise analysis for medication development and quality control. Each detector, including the Flame Ionization Detector and Mass Spectrometer, is examined for its distinct capabilities and importance in adhering to regulatory standards. This adherence not only enhances the reliability of pharmaceutical products but also ensures their safety for consumer use. By understanding the functionality of these detectors, professionals can better appreciate their contributions to the pharmaceutical industry.
Introduction
The pharmaceutical industry is increasingly reliant on sophisticated analytical techniques to ensure the safety and efficacy of drug products. Central to these efforts are chromatography detectors, which are essential tools that enhance the precision of chemical analyses in laboratories. This article explores ten pivotal chromatography detectors that are shaping the future of pharmaceutical analysis, examining their unique capabilities and the critical roles they play in meeting regulatory standards. As the demand for accurate and reliable testing methods escalates, how do these detectors adapt to the evolving landscape of pharmaceutical development?
JM Science: High-Performance Chromatography Detectors for Accurate Analysis
JM Science Inc. provides a wide range of high-performance chromatography detectors that are specifically designed to fulfill the stringent requirements of pharmaceutical laboratories. These detectors play a crucial role in ensuring precise examinations, which are essential for medication development and quality control. The chromatography instrumentation market is projected to experience significant growth in 2025, with chromatography detectors at the forefront due to their capacity to enhance analytical precision and reliability. Notably, advancements in Ultra-Performance Liquid Chromatography (UPLC) have demonstrated improved sensitivity and faster evaluation times, directly benefiting pharmaceutical accuracy. Furthermore, North America is anticipated to be the fastest-growing market for chromatography instrumentation and chromatography detectors during the forecast period, reflecting the rising demand for these sophisticated tools.
Industry leaders emphasize the critical nature of precise analysis in medication development. Bryan Abney, a prominent figure in quality assurance, asserts, "Quality is essential at every stage of development, from initial research to final product distribution." This perspective resonates throughout the industry, highlighting the necessity of reliable instrumentation to uphold high-quality standards. Additionally, the FDA's FY2023 report underscores ongoing quality-related challenges within the drug industry, reinforcing the urgent need for dependable analytical tools.
The practical applications of chromatography detectors demonstrate their significant impact on drug analysis. For instance, the incorporation of advanced auto-sampler accessories has markedly enhanced sample introduction efficiency, resulting in more consistent laboratory outcomes. As the pharmaceutical industry continues to evolve, the demand for high-performance chromatography detectors remains essential, ensuring laboratories can meet regulatory standards and improve patient safety through rigorous quality control.
Flame Ionization Detector (FID): The Standard for Gas Chromatography
The Flame Ionization Detector (FID), a type of chromatography detector, stands as the gold standard in gas chromatography, renowned for its exceptional sensitivity to hydrocarbons. Operating by combusting the sample in a hydrogen flame, the FID produces hydronium ions (CHO+) that are attracted to a polarized collector, generating a measurable current. This mechanism renders the FID as one of the chromatography detectors particularly adept at analyzing volatile organic compounds (VOCs), making it extensively utilized in pharmaceutical laboratories for its reliability and accuracy in quantifying trace levels of substances.
Pharmaceutical lab managers consistently emphasize the performance of chromatography detectors, particularly the FID, citing its unparalleled ability to detect VOCs with precision. During drug development, for instance, the FID has proven invaluable in analyzing the purity of compounds, ensuring compliance with stringent regulatory standards. One lab manager remarked, "The FID is our go-to detector for VOC analysis; its reliability is unmatched in our processes."
Real-world applications illustrate that the FID effectively identifies and quantifies VOCs, facilitating the development of safer and more effective medicinal products. Furthermore, the sensitivity of the FID extends to hydrocarbons, enabling the detection of even minute concentrations. This capability is essential in drug-related environments, where trace contaminants can significantly affect product quality and security. Consequently, the FID and other chromatography detectors remain indispensable tools for laboratories committed to maintaining high standards in their analytical processes, particularly when compared to other detectors that may not offer the same level of sensitivity and reliability.
Mass Spectrometer (MS): Advanced Detection for Complex Samples
Mass Spectrometry (MS) serves as a cornerstone in drug laboratories, providing unparalleled detection capabilities for complex samples. By analyzing the mass-to-charge ratio of ions, MS enables the precise identification and quantification of compounds, which is crucial for characterizing pharmaceutical formulations and ensuring their efficacy.
This technology excels at detecting impurities and conducting pharmacokinetic studies, thereby playing a vital role in the development of reliable medications. Researchers emphasize that the accuracy rates of MS in formulation assessment significantly enhance the dependability of results, establishing it as an indispensable instrument in the pharmaceutical industry.
A clinical trial has demonstrated considerable advancements in medication formulation evaluation, underscoring MS's effectiveness in upholding safety standards. The integration of MS into drug formulation characterization not only streamlines the analysis process but also supports regulatory compliance, ultimately propelling the advancement of medical sciences.
Thermal Conductivity Detector (TCD): Versatile Detection for Various Compounds
The Thermal Conductivity Detector (TCD) is an indispensable instrument among chromatography detectors, distinguished by its proficiency in measuring the thermal conductivity of gases. This adaptability allows for the effective identification of permanent gases and low-boiling compounds, making it particularly valuable in drug laboratories where a variety of sample types are analyzed. TCDs are frequently utilized in environmental monitoring, ensuring compliance with rigorous regulatory standards for medical products.
As we look to 2025, the applications of TCDs in drug laboratories are broadening, with their capacity to analyze low-boiling compounds standing out as particularly significant. TCDs are capable of detecting both organic and inorganic gases, a critical aspect of quality control processes. Industry experts underscore the ability of chromatography detectors, such as the TCD, to deliver a universal response, rendering them suitable for a diverse array of compounds, thereby enhancing their functionality across various laboratory environments.
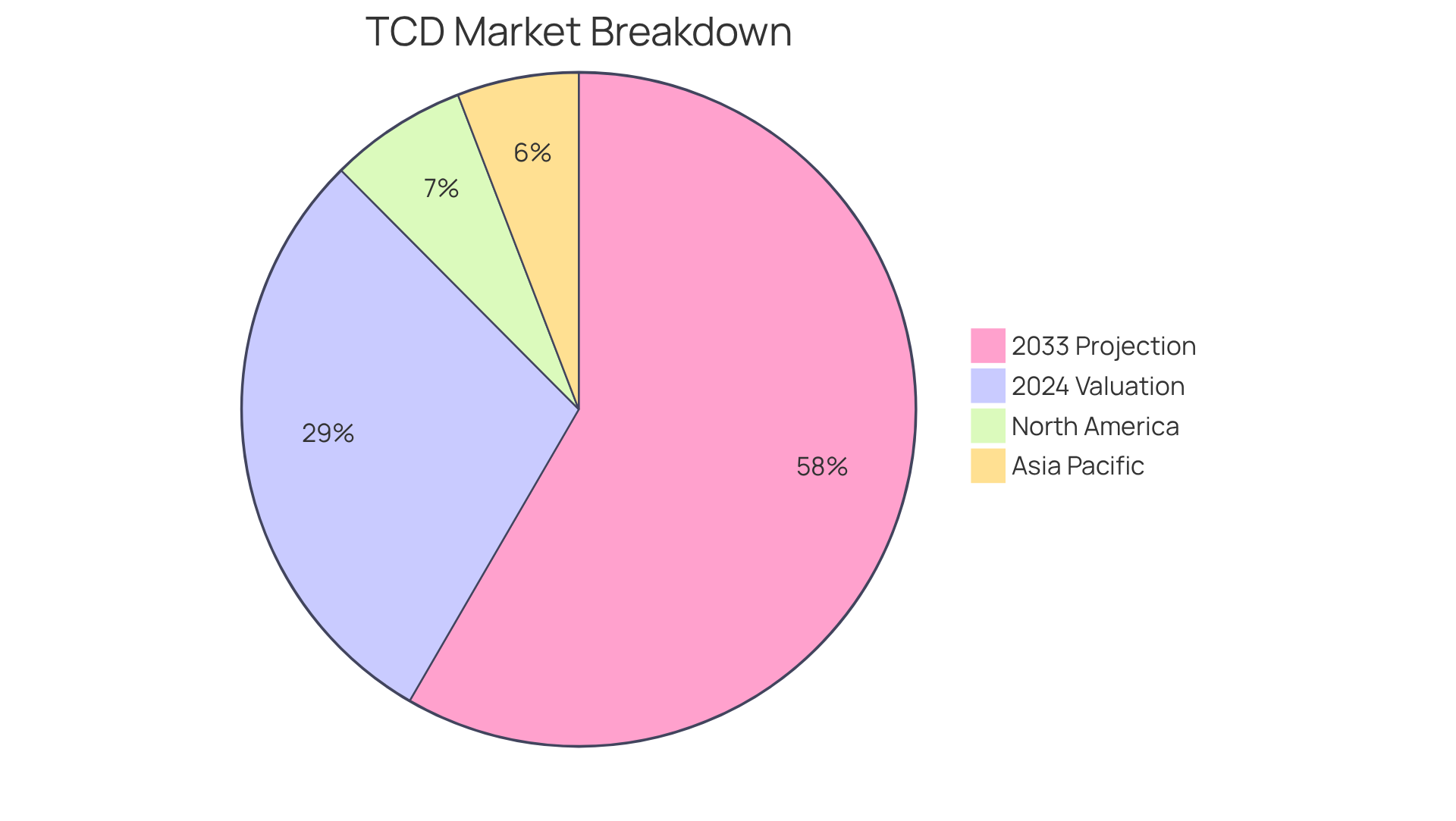
Practical examples illustrate the TCD's contribution to environmental monitoring in the pharmaceutical sector, aiding in the assessment of compliance with environmental regulations. The TCD market was valued at USD 150 million in 2024, with projections indicating substantial growth to USD 300 million by 2033, highlighting the increasing demand for precise gas evaluation across multiple industries. This expansion is driven by technological advancements and the need for reliable analytical tools in medical applications. Furthermore, in 2023, North America accounted for 34% and Asia Pacific for 30% of the TCD market, underscoring the regional significance of this technology.
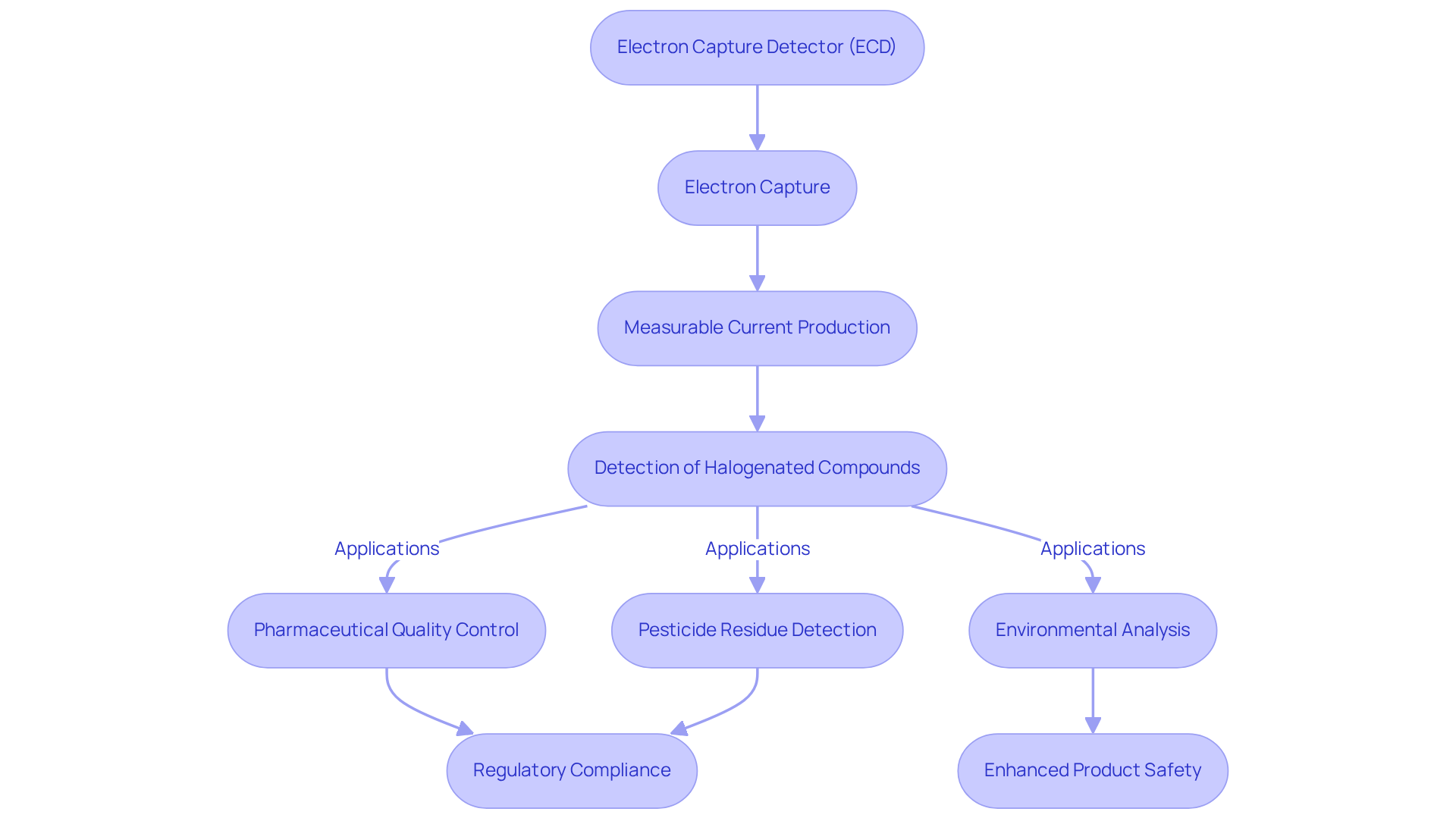
Electron Capture Detector (ECD): Essential for Halogenated Compound Detection
Chromatography detectors, such as the Electron Capture Detector (ECD), serve as pivotal instruments in the identification of halogenated compounds, proving indispensable in drug evaluation. By capturing electrons from the sample, the ECD produces a measurable current that indicates the presence of halogens. This capability is crucial for the assessment of pesticides, herbicides, and various halogenated compounds, thereby ensuring that medicinal products adhere to stringent security and regulatory standards as evaluated by chromatography detectors.
Recent regulations have heightened the focus on halogenated substances in pharmaceuticals. In 2021, 14 out of 50 new chemical entities approved by the FDA contained halogens, including four with chlorine and two featuring a combination of fluorine and chlorine. The sensitivity and selectivity of chromatography detectors, such as the ECD, for electronegative compounds render it particularly effective in this arena, as it can detect even trace amounts of halogenated contaminants.
Real-world applications of the ECD underscore its effectiveness in maintaining pharmaceutical quality standards. For example, chromatography detectors such as the ECD are utilized in environmental analysis and pesticide residue detection, where their capacity to identify halogenated compounds is vital. Research indicates that the ECD achieves outstanding results in detecting these compounds, significantly reducing false positives and enhancing the reliability of testing.
Pharmaceutical analysts emphasize the importance of chromatography detectors, such as the ECD, in ensuring compliance with regulatory standards. Notably, the ECD's design incorporates a reference channel that compensates for background signals, guaranteeing accurate detection of analytes. This precision is essential for laboratories striving to meet the evolving demands of drug efficacy and reliability. James Lovelock, the inventor of the ECD in 1957, highlighted its role in detecting electron-absorbing halogenated compounds, affirming its enduring significance in the field.
In summary, chromatography detectors, such as the ECD, not only play a critical role in identifying halogenated compounds but also assist the pharmaceutical industry in meeting regulatory standards, ultimately contributing to the safety and efficacy of medicinal products. For laboratory managers within the drug industry, effectively leveraging the ECD can enhance compliance and ensure the integrity of their analyses.
Photoionization Detector (PID): Key for Volatile Organic Compound Detection
The Photoionization Detector (PID) serves as an essential instrument for detecting volatile organic compounds (VOCs) in gas chromatography. By utilizing ultraviolet light to ionize the sample, it enables the detection of low concentrations of VOCs, a capability that is particularly critical in drug laboratories. Here, monitoring VOC levels is vital for ensuring product integrity and compliance with environmental regulations. The minimum detection limits for various sensor variants differ, with some reaching as low as 1 ppb for the white + gold spot variant, while others have limits of:
- 5 ppb for unspecified colors
- 20 ppb for the red electrode stack
- 500 ppb for the blue electrode stack, among others.
Effective VOC monitoring transcends regulatory compliance; it represents a moral imperative for drug companies. Emissions of VOCs, especially Dichloromethane (DCM), pose significant health risks, including dizziness and respiratory irritation, and contribute to environmental challenges such as ground-level ozone and smog. The Tiger 11.7 eV gas detector exemplifies a premier solution for detecting DCM, as evidenced by Hovione, a leading company that has successfully leveraged this technology to identify leaks and enhance worker protection.
The significance of PIDs extends beyond mere compliance; they are instrumental in maintaining operational efficiency and safeguarding public health. As environmental compliance officers emphasize, continuous monitoring of VOC levels is crucial for mitigating risks associated with drug manufacturing processes. By adopting advanced PID technology, laboratories can ensure they adhere to regulatory standards while prioritizing the protection of their products and the environment. Furthermore, customers can take advantage of a limited-time trade-in offer to upgrade to the latest Tiger model, thereby enhancing their detection capabilities.
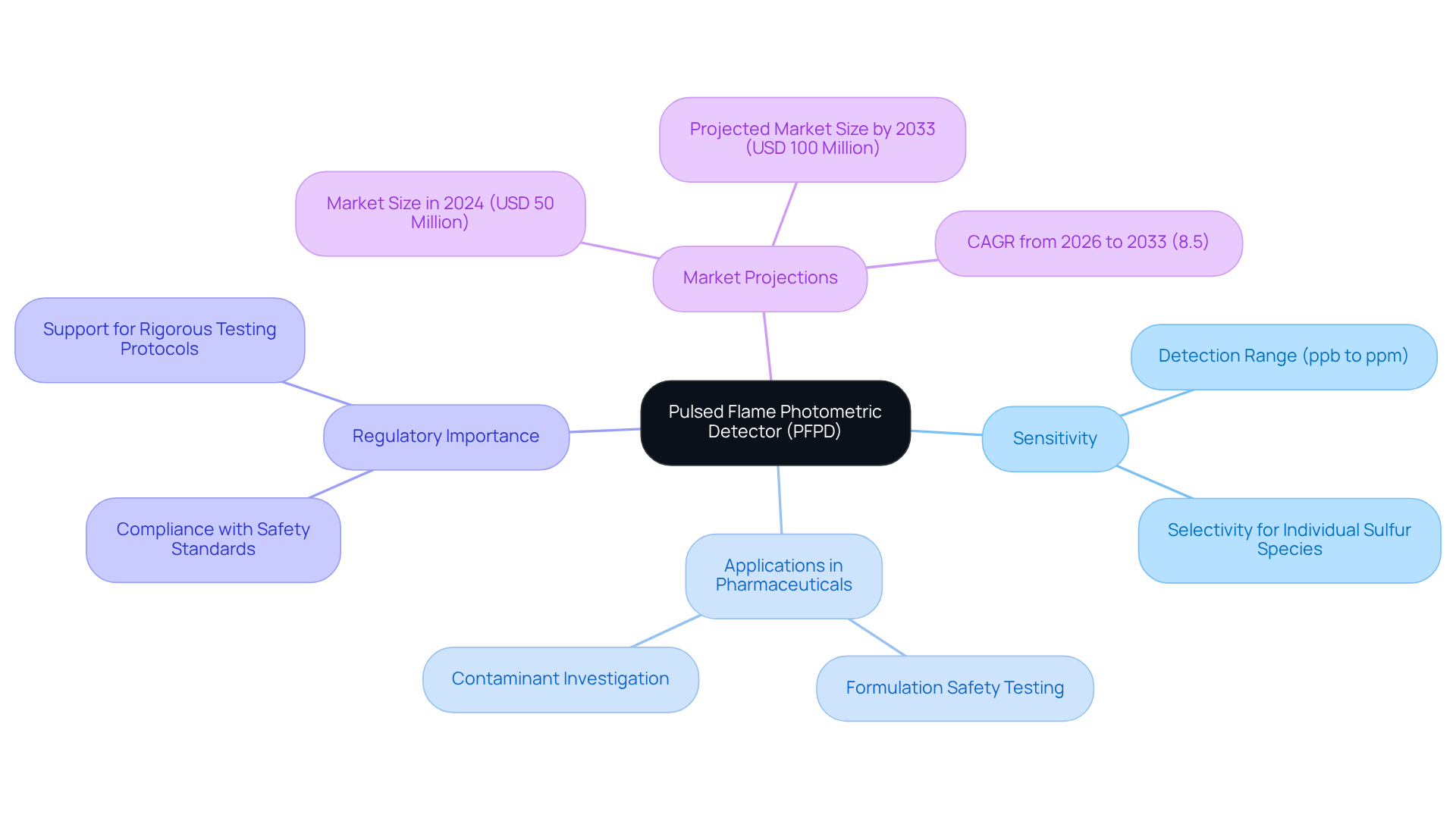
Pulsed Flame Photometric Detector (PFPD): Specialized Detection for Sulfur and Phosphorus
The Pulsed Flame Photometric Detector (PFPD) stands as a cutting-edge instrument meticulously crafted for the detection of sulfur and phosphorus compounds, rendering it essential in pharmaceutical analysis. By employing a pulsing flame mechanism, the PFPD markedly enhances sensitivity, facilitating the detection of trace levels of these vital elements. This capability proves particularly beneficial for scrutinizing medication formulations that may contain sulfur or phosphorus, thereby ensuring compliance with the security and effectiveness standards mandated by regulatory agencies.
Pharmaceutical chemists have recognized the PFPD's remarkable sensitivity, which permits the selective measurement of individual sulfur species ranging from low parts per billion (ppb) to parts per million (ppm) levels. In practical applications, the PFPD has successfully identified sulfur and phosphorus across various medication formulations, thereby supporting rigorous testing protocols.
Recent research underscores the PFPD's pivotal role in formulation safety testing, where it is employed to investigate potential contaminants and guarantee that medicinal products adhere to stringent regulatory standards. Its applications transcend mere detection; the PFPD is also utilized in comprehensive drug formulation evaluation, supplying critical data that bolsters the development of safe and effective medications. As highlighted by Verified Market Reports, the market for PFPDs is projected to expand significantly, reaching an estimated USD 100 million by 2033, underscoring the importance of this technology in drug laboratories.
Atomic Emission Detector (AED): Unique Elemental Analysis Capabilities
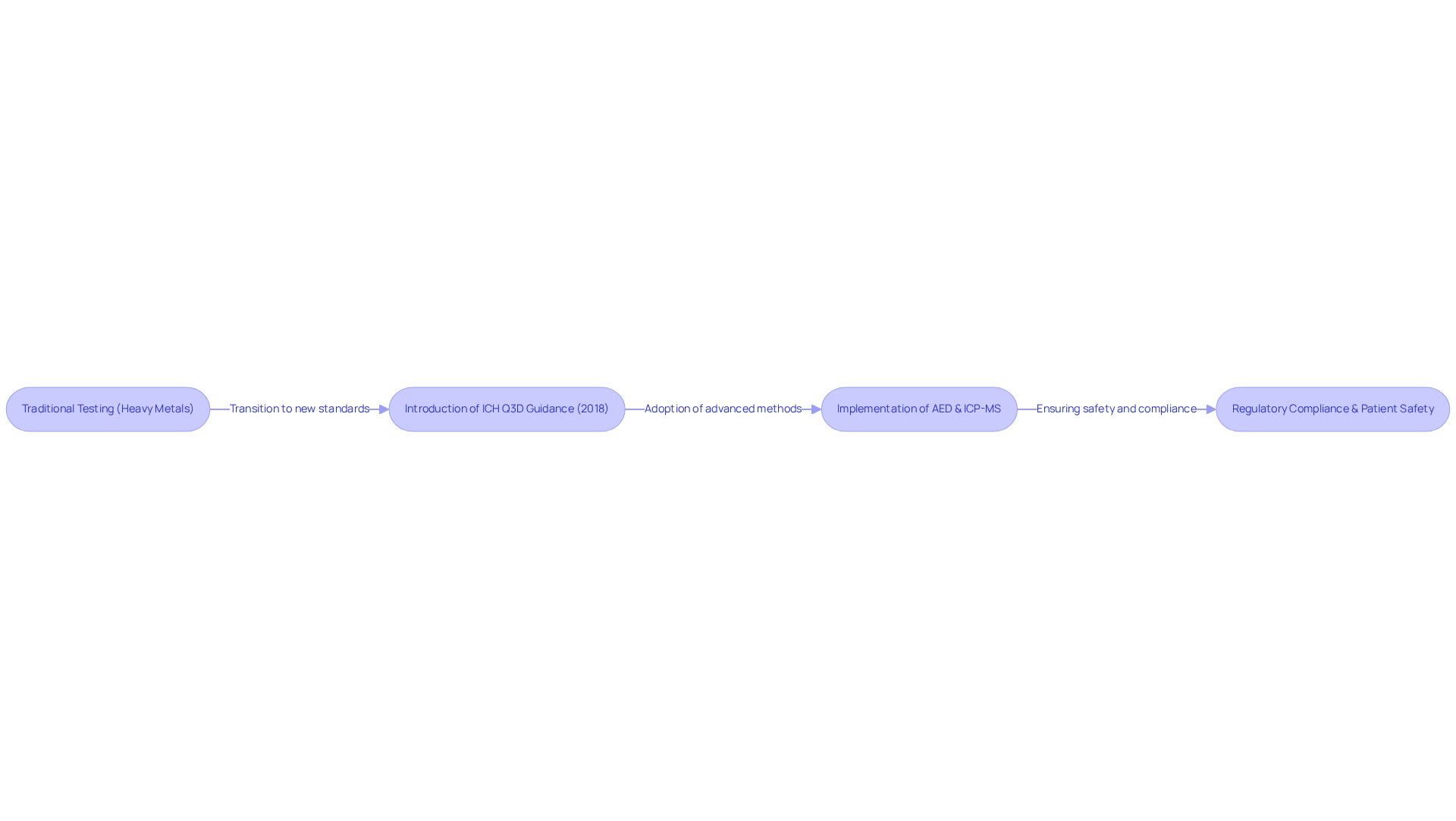
The Atomic Emission Detector (AED) serves as a powerful instrument for elemental evaluation in chromatography detectors, uniquely capable of detecting elements through their characteristic emission spectra. This capability is essential in laboratory settings, where ensuring that formulations are devoid of harmful levels of elemental contaminants is crucial for patient well-being. Since the implementation of stringent testing requirements in 2018, following the ICH Q3D guidance, the focus on elemental impurities has intensified. These regulations mandate that pharmaceutical products undergo thorough examination to confirm accurate dosing and the absence of contaminants.
Recent research underscores the critical role of elemental analysis in medication security and compliance. Historically, elemental testing often relied on a colorimetric 'Heavy Metals' test, which could pass with 500 parts per million (ppm) of mercury in a sample. The transition to advanced methods such as inductively coupled plasma-mass spectroscopy (ICP-MS) has revolutionized the detection of elemental impurities, enabling measurements at parts-per-billion (ppb) concentrations. This shift not only enhances the precision of testing but also aligns with the allowable daily exposure (PDE) limits established for 24 elements in the ICH Q3D document, thereby prioritizing patient well-being.
Real-world applications of chromatography detectors in drug laboratories illustrate their effectiveness in safeguarding formulations. By accurately identifying and quantifying metals and metalloids, chromatography detectors play a crucial role in ensuring compliance with regulations. Regulatory organizations emphasize the importance of such analytical methods, noting that they significantly contribute to preserving the integrity of medical products. As Alan Cross from RSSL stated, "Any analytical testing conducted on medicinal and biological substances is primarily to guarantee that patient well-being is upheld." As the sector continues to evolve, the integration of sophisticated chromatography detectors, such as the AED, will remain vital in the ongoing effort to enhance medication security and effectiveness.
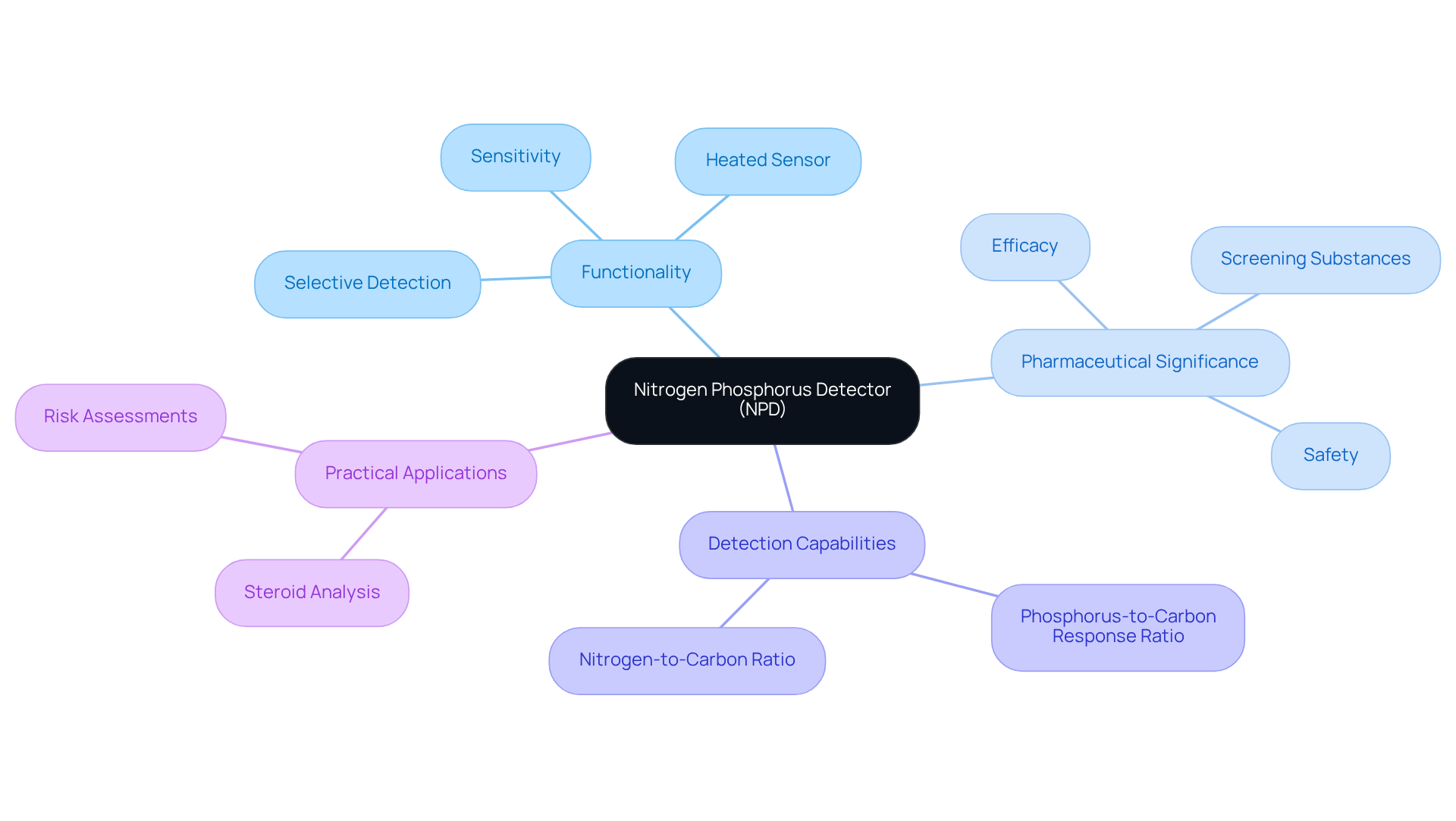
Nitrogen Phosphorus Detector (NPD): Tailored Detection for Nitrogen and Phosphorus Compounds
The Nitrogen Phosphorus Detector (NPD) is an essential type of chromatography detector that plays a crucial role in detecting nitrogen and phosphorus compounds in gas chromatography. By utilizing a heated sensor, it enhances sensitivity to these elements, making it particularly advantageous for analyzing substances containing nitrogenous or phosphorous compounds. This specialized detection capability is vital for ensuring the safety and efficacy of pharmaceutical products.
The NPD is indispensable in screening substances, given that most medications contain nitrogen, underscoring its significance in pharmaceutical applications. Recent advancements in NPD technology have further amplified its effectiveness in pharmaceutical evaluations. The NPD achieves phosphorus-to-carbon response ratios of up to 50,000:1 and nitrogen-to-carbon ratios of 5,000:1, enabling precise measurements even in complex mixtures. This selectivity proves especially beneficial in toxicological studies, where the accurate detection of nitrogen-containing compounds is paramount, allowing for their identification without interference from co-eluting substances.
Pharmaceutical researchers emphasize the critical role of chromatography detectors in ensuring medication safety, noting that their heightened sensitivity makes them a preferred choice for analyzing substances and metabolites. For example, John William Honour articulates, "compounds containing nitrogen atoms can be detected with a nitrogen-phosphorus detector (NPD) but as most steroids commonly encountered do not contain nitrogen, to use this detection system requires formation of nitrogen containing derivatives such as methyloximes." This statement illustrates the NPD's ability to deliver reliable results in challenging analytical contexts.
In practical applications, the NPD has proven effective in analyzing steroid compounds, which frequently necessitate the creation of nitrogen-containing derivatives for accurate detection. This methodology enhances the reliability of steroid measurements, facilitating improved risk assessments in pharmaceutical development. Ultimately, the NPD emerges as an essential instrument in the healthcare sector, ensuring that medication products adhere to rigorous safety and efficacy standards.
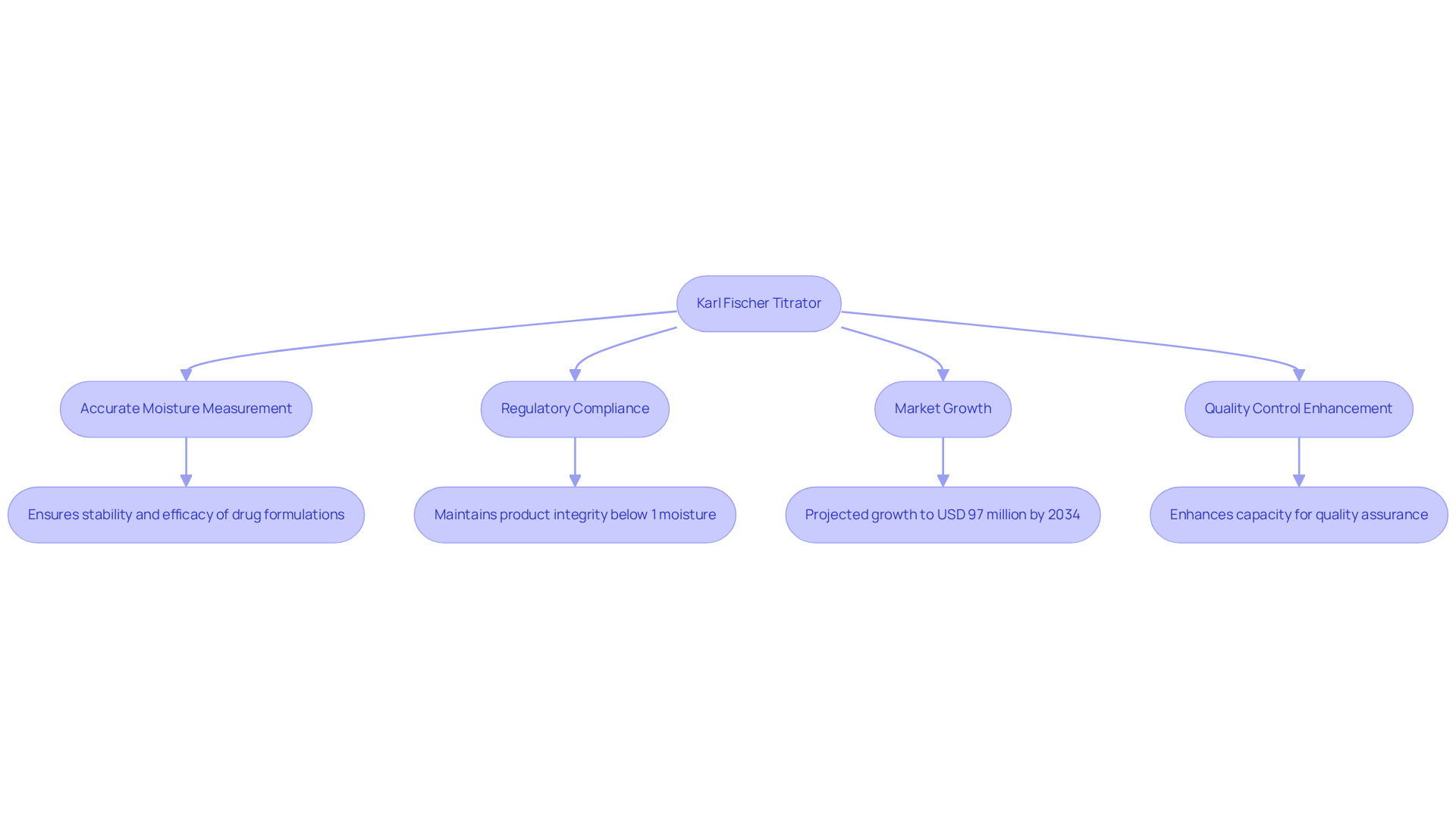
Karl Fischer Titrator by JM Science: Innovative Tool for Moisture Analysis
The Karl Fischer Titrator from JM Science represents an advanced solution for moisture evaluation in drug products. This titrator employs a precise chemical reaction to accurately measure water content, a critical factor in ensuring the stability and efficacy of drug formulations. Precise moisture assessment is vital; even slight variations can compromise the quality and effectiveness of active medicinal ingredients (APIs). For example, drug manufacturers must control moisture content to less than 1% for solid oral dosage forms, ensuring compliance with regulatory standards and maintaining product integrity.
The AQ-300 Coulometric Karl Fischer Titrator and the Hiranuma Aquacounter AQV-300 Volumetric Titrator exemplify JM Science's commitment to delivering premium scientific instruments that adhere to the Japanese Pharmacopoeia. These titrators are indispensable for pharmaceutical and medicine testing, ensuring accurate moisture level measurements that uphold product quality. Moreover, these products are conveniently available through JM Science's e-commerce platform, facilitating access to high-quality titration solutions for laboratories.
Statistics indicate that the U.S. Karl Fischer titrators market generated USD 79.8 million in 2024, with projections forecasting a growth rate of 2% CAGR, potentially reaching USD 97 million by 2034. This growth underscores the increasing reliance on moisture analysis within the drug industry. Quality control managers have observed that JM Science's Karl Fischer Titrator significantly enhances their capacity to ensure product quality and compliance, solidifying its position as an essential tool in contemporary laboratories.
Furthermore, the method's unparalleled accuracy and specificity establish it as the gold standard for moisture content determination, reinforcing its significance in drug stability and efficacy. By delivering reliable moisture measurements, JM Science's Karl Fischer Titrator plays a pivotal role in enabling laboratories to maintain the highest standards of quality control in pharmaceutical manufacturing.
Conclusion
The significance of chromatography detectors in pharmaceutical laboratories is paramount. These advanced instruments are essential for ensuring the accuracy and reliability of drug analysis, a critical component of medication development and quality control. As the pharmaceutical industry continues to evolve, the demand for high-performance chromatography detectors is on the rise, driven by the need for precise analysis and adherence to stringent regulatory standards.
This article has explored various types of chromatography detectors, including:
- Flame Ionization Detector (FID)
- Mass Spectrometer (MS)
- Thermal Conductivity Detector (TCD)
- Electron Capture Detector (ECD)
- Photoionization Detector (PID)
- Pulsed Flame Photometric Detector (PFPD)
- Atomic Emission Detector (AED)
- Nitrogen Phosphorus Detector (NPD)
- Karl Fischer Titrator
Each of these detectors presents unique advantages tailored to specific applications within pharmaceutical analysis, ranging from the detection of volatile organic compounds to the measurement of moisture content. The advancements in these technologies not only enhance analytical precision but also support regulatory compliance and ensure patient safety.
With the market for chromatography detectors projected to expand significantly by 2025, it is imperative for pharmaceutical laboratories to adopt these innovative tools. By investing in state-of-the-art chromatography detectors, laboratories can guarantee high-quality analyses that uphold the integrity of medicinal products. Embracing these advancements will enhance operational efficiency and reinforce the commitment to safety and efficacy in drug development, ultimately benefiting patients and the broader healthcare landscape.




