Overview
The article titled "10 Essential Process Analyzers for Pharmaceutical Labs" highlights crucial tools and technologies that significantly enhance analytical capabilities within pharmaceutical laboratories. While the content does not explicitly enumerate these analyzers, it delves into advanced instruments and solutions, including:
- HPLC systems
- Mass spectrometers
- Karl Fischer titrators
These discussions collectively emphasize the critical role of precise measurement and analysis in ensuring drug quality and compliance with industry standards.
Introduction
The pharmaceutical industry stands at the forefront of technological advancements, where process analyzers play a pivotal role in ensuring the quality and safety of medications. As laboratories strive to meet stringent regulatory standards and enhance their analytical capabilities, selecting the right tools becomes crucial. This article delves into ten essential process analyzers that not only streamline laboratory workflows but also elevate the accuracy and reliability of pharmaceutical analysis. With a myriad of options available, laboratories must consider how to determine which analyzers will best meet their evolving needs.
JM Science HPLC Solutions: Precision in Process Analysis
JM Science presents a robust array of HPLC solutions meticulously crafted to satisfy the stringent requirements of drug evaluation. These high-performance systems incorporate cutting-edge features that ensure precise separation and quantification of compounds, which are crucial for both quality control and research applications. The integration of innovative technologies not only accelerates analysis times but also enhances reproducibility—elements that are vital in the ever-evolving pharmaceutical landscape. Moreover, JM Science enriches the user experience by providing extensive support resources, including:
- Application libraries
- Instructional videos
This empowers laboratories to maximize the potential of their HPLC systems. As the HPLC market continues to grow, driven by increasing demands for superior medications and effective analytical methods, JM Science remains steadfast in its dedication to delivering exceptional solutions that address the industry's shifting requirements.
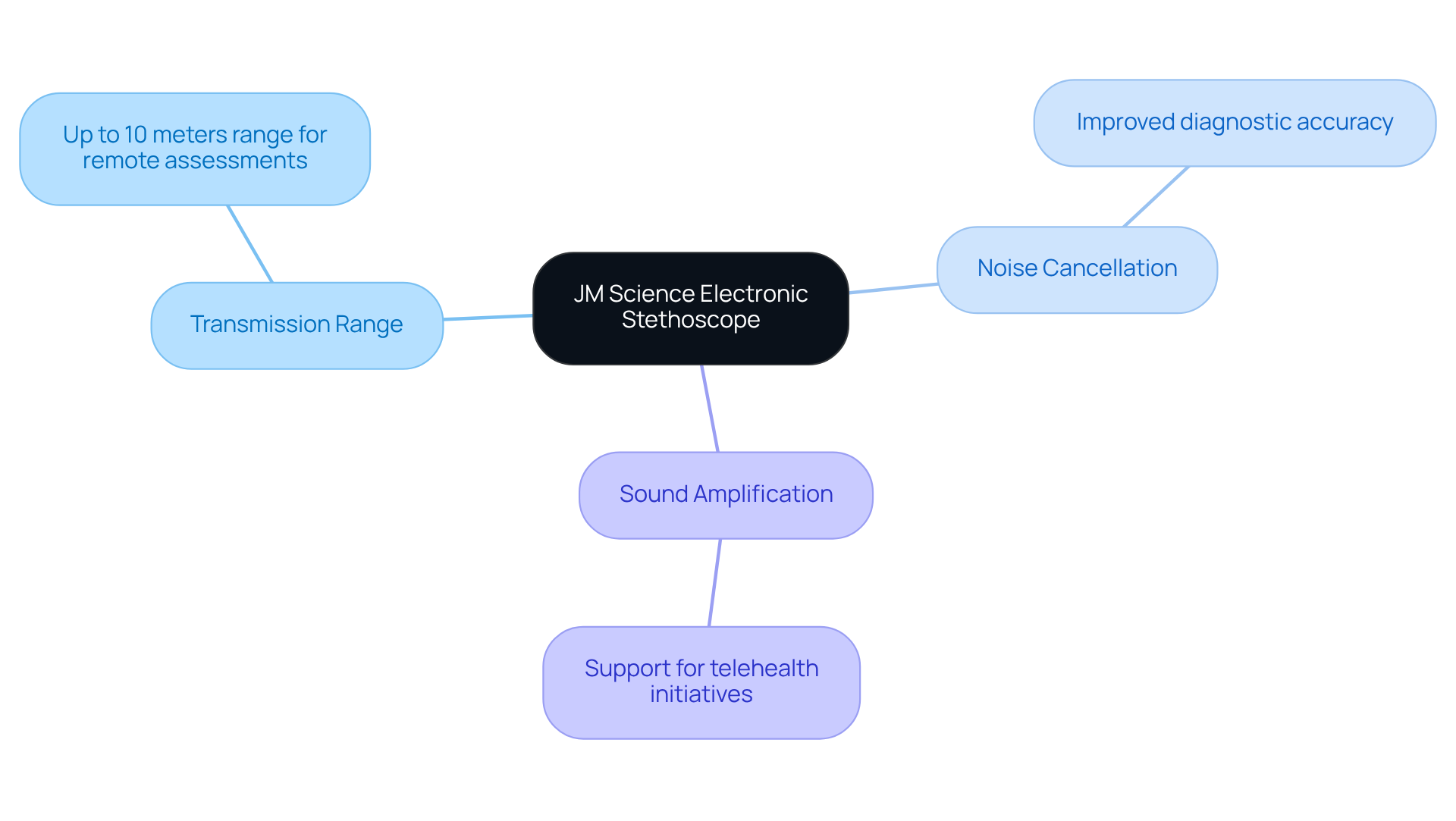
JM Science Electronic Stethoscope: Advancing Remote Patient Monitoring
The JM Science electronic stethoscope represents a significant advancement in remote patient monitoring technology. This innovative device empowers healthcare providers to listen to heart and lung sounds from a distance, thereby enhancing the quality of patient assessments. With a transmission range of up to 10 meters, it allows for comprehensive examinations without necessitating physical proximity, which is crucial in today’s healthcare landscape.
Equipped with cutting-edge features such as noise cancellation and sound amplification, this stethoscope significantly improves diagnostic accuracy. These enhancements make it an indispensable tool for modern healthcare practices, facilitating thorough evaluations while supporting telehealth initiatives. As a result, it ensures continuous patient care and streamlines the assessment process, ultimately benefiting both patients and providers.
In summary, the JM Science electronic stethoscope not only exemplifies technological innovation but also addresses the growing need for effective remote monitoring solutions. By integrating this advanced device into healthcare routines, providers can enhance their diagnostic capabilities, ensuring that patient care remains at the forefront of medical practice.
JM Science Karl Fischer Titrators: Essential for Moisture Analysis
JM Science presents an extensive range of Karl Fischer titrators, essential for the accurate determination of moisture content in pharmaceutical products. These titrators utilize a highly precise chemical reaction to quantify water content, thereby ensuring compliance with industry standards. Notably, the AQ-300 Coulometric Karl Fischer Titrator delivers swift and reliable results, making it suitable for both routine assessments and advanced research applications. By employing these titrators, laboratories can uphold the standard and stability of their products, which is vital for ensuring patient safety.
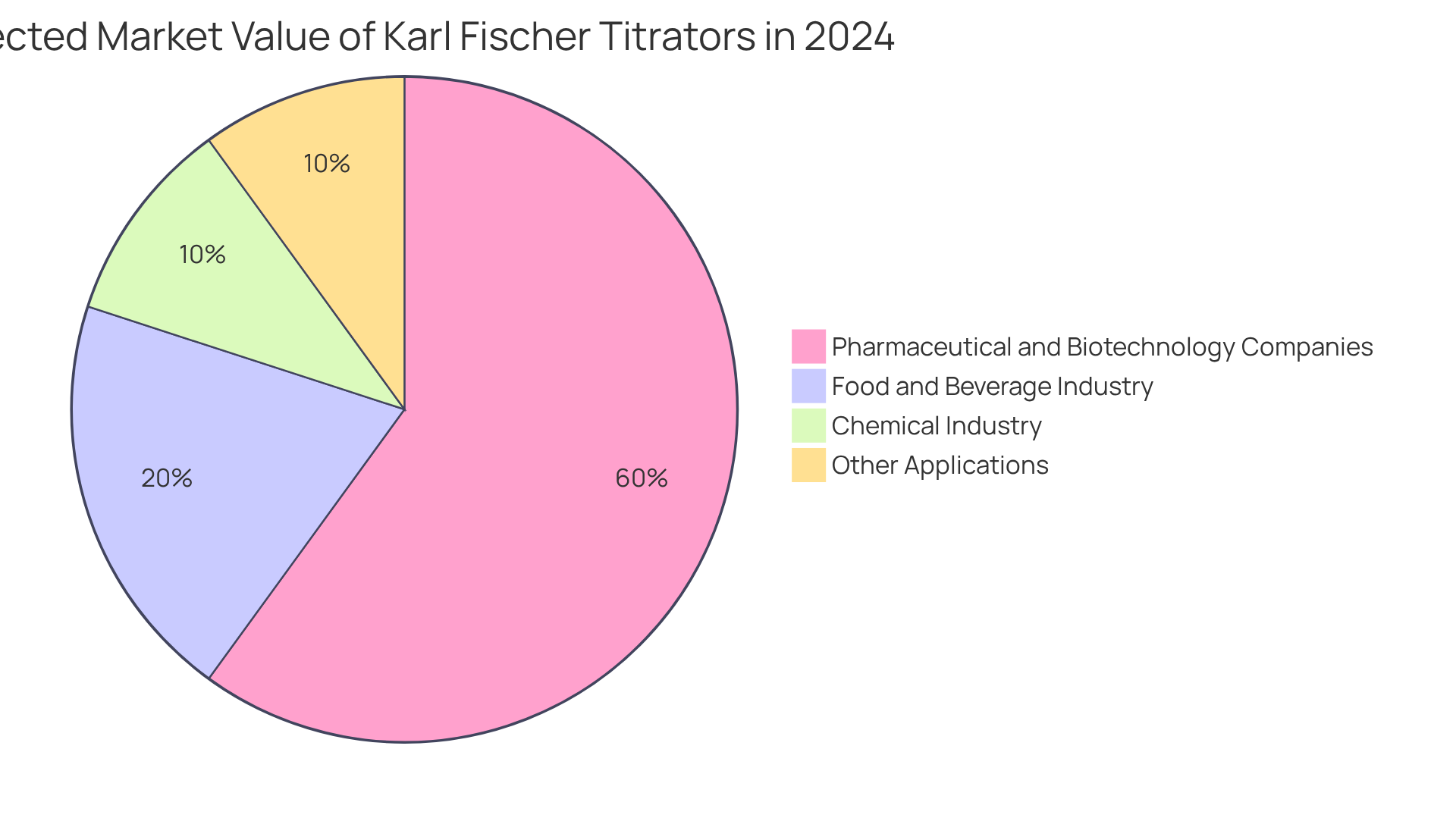
The global market for Karl Fischer titrators was projected to reach approximately USD 225.2 million in 2024, with forecasts indicating a growth rate of 3.1% CAGR through 2034. This growth is driven by the increasing demand for moisture evaluation in drug manufacturing, underscoring the critical role of moisture content assessment in guaranteeing drug safety and efficacy. Even minor fluctuations can significantly impact product standards, highlighting the importance of precise measurements.
As industry experts emphasize, the Karl Fischer titration technique remains the gold standard for moisture assessment, reinforcing its pivotal role in drug manufacturing oversight. The reliability and accuracy of these titrators not only support regulatory compliance but also enhance the overall quality of pharmaceutical products, ultimately contributing to improved patient outcomes.
Agilent Technologies Chromatography Systems: Industry Standard for Analysis
Agilent Technologies offers a comprehensive suite of chromatography systems that are recognized as the industry standard for pharmaceutical analysis. These systems are designed for high throughput and accuracy, making them suitable for a wide range of applications, from drug development to quality assurance.
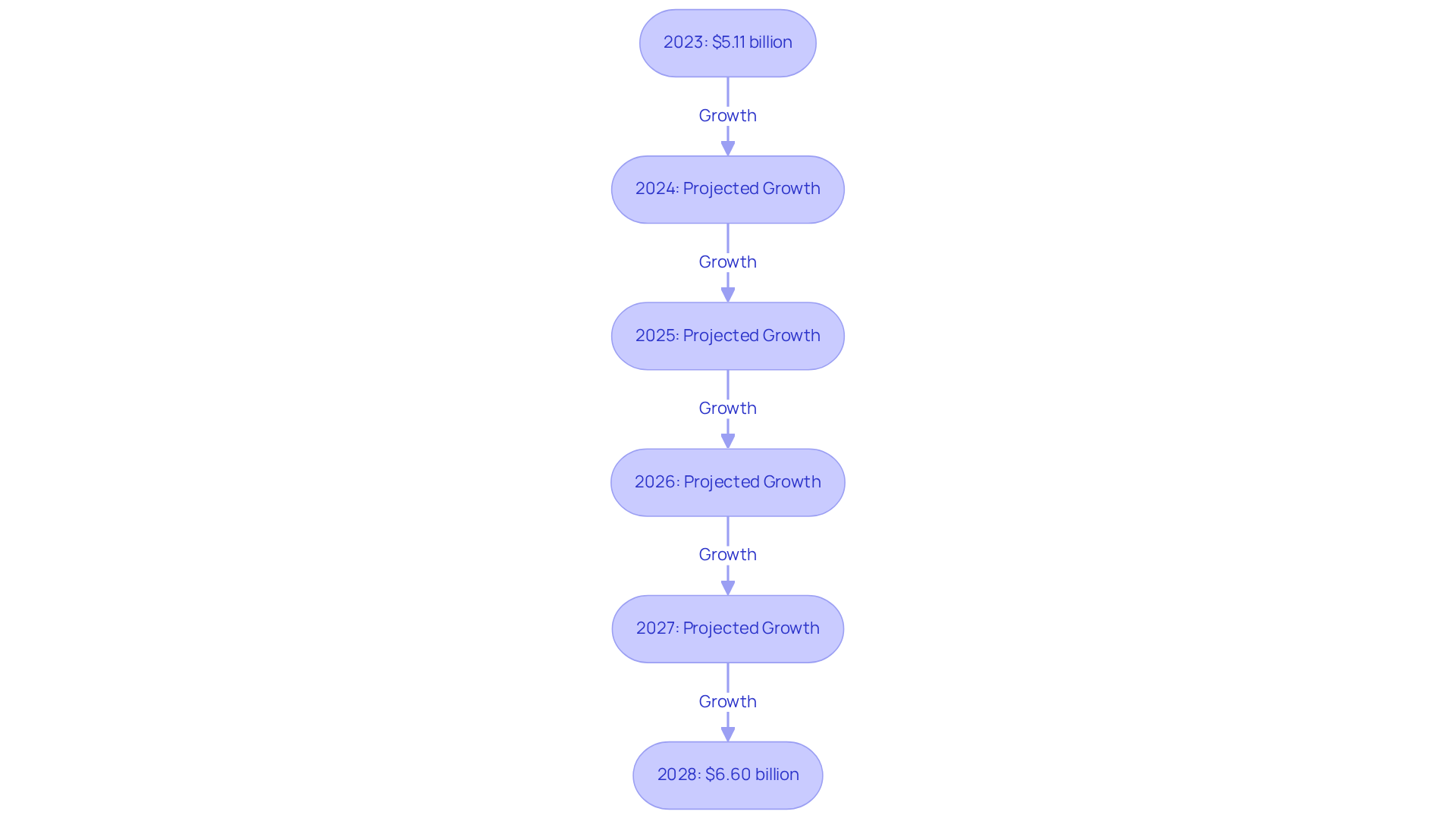
In 2023, the chromatography market generated revenues of $5.11 billion and is projected to grow to $6.60 billion by 2028, highlighting the significance of Agilent's systems within this expanding market. Features such as advanced data management software and customizable configurations enhance workspace efficiency and ensure compliance with regulatory requirements.
By integrating Agilent's chromatography systems, facilities can achieve consistent and reliable results, which are crucial for maintaining product quality. Furthermore, the incorporation of cutting-edge technologies, including AI and machine learning into chromatography software, is enhancing analytical capabilities within the pharmaceutical industry.
As Sudharshana Seshadri, vice president and general manager of Agilent’s Liquid Chromatography, Mass Spectrometry, and Automation Divisions, asserts, "The new 6495D LC/TQ offers significant improvements in sensitivity and speed over the current 6495C, which is already a reputable high-end LC/TQ." This statement underscores Agilent's unwavering commitment to innovation and excellence in analytical instrumentation.
Thermo Fisher Scientific Laboratory Equipment: Supporting Process Analysis
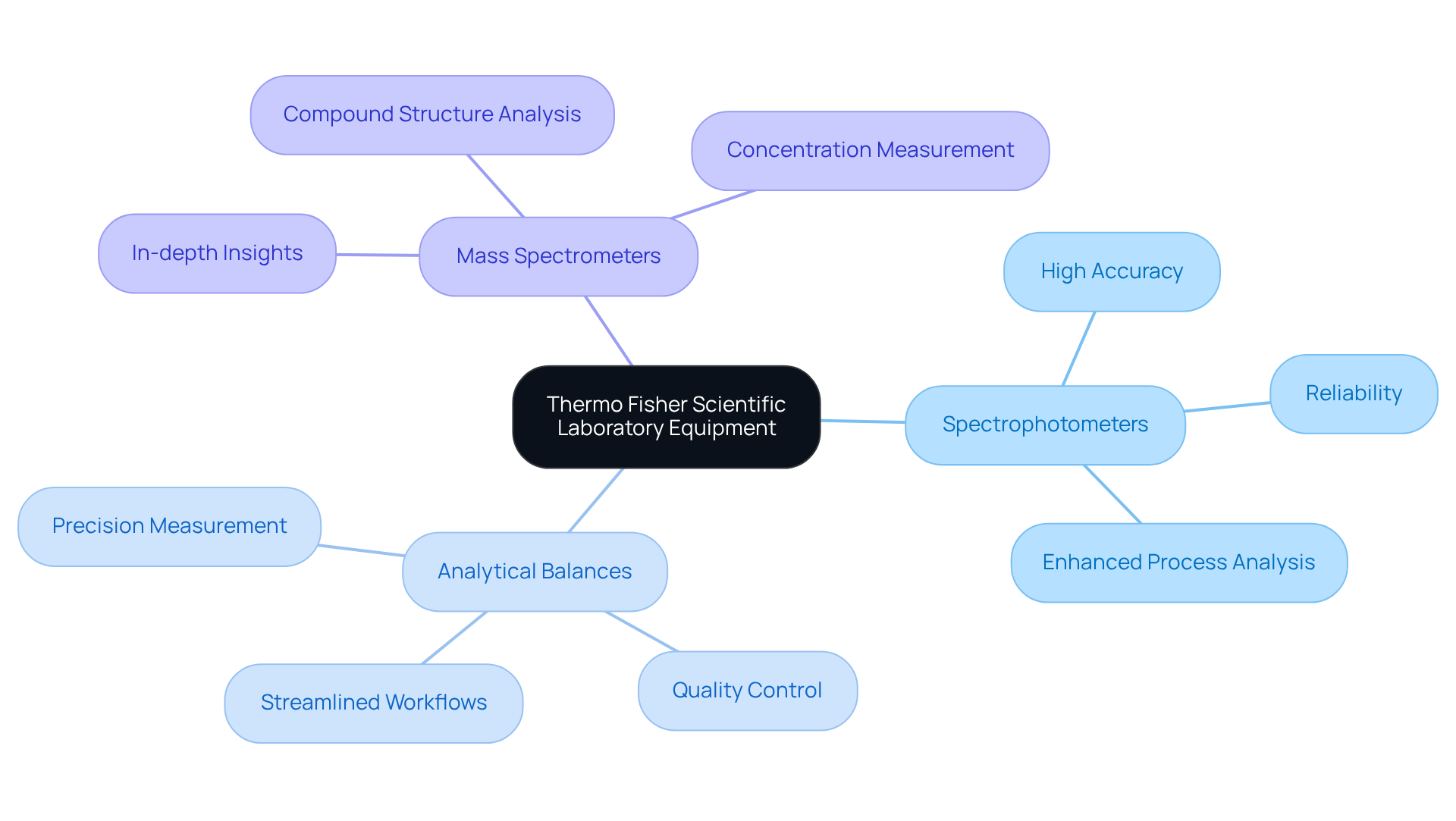
Thermo Fisher Scientific stands as a leader in the supply of laboratory equipment that significantly enhances process analyzers in drug development environments. Their advanced spectrophotometers, analytical balances, and mass spectrometers are meticulously engineered for high accuracy and reliability—qualities that are crucial for adhering to the stringent standards of the pharmaceutical industry. Notably, their mass spectrometry systems provide in-depth insights into compound structures and concentrations, which are essential for both drug development and quality control processes.
By leveraging Thermo Fisher's sophisticated process analyzers, research facilities can elevate their analytical capabilities, ensuring compliance with rigorous regulatory requirements and enhancing research outcomes. This integration of advanced technology not only streamlines workflows but also addresses the evolving needs of drug research, ultimately contributing to the development of safer and more effective therapeutics.
Mettler Toledo Analytical Balances: Accuracy in Measurements
Mettler Toledo analytical balances are meticulously engineered to deliver exceptional precision and dependability, rendering them indispensable in pharmaceutical environments. These balances excel at measuring small sample sizes with remarkable accuracy, a critical requirement for drug formulation and control testing.
With features such as integrated calibration and anti-draft enclosures, they ensure reliable performance, even under demanding testing conditions. By incorporating Mettler Toledo balances into their operational processes, facilities can significantly enhance measurement precision, ultimately contributing to the superior standard of medical products.
PerkinElmer Spectrophotometers: Key for Quantitative Analysis
PerkinElmer spectrophotometers play a critical role in pharmaceutical settings, particularly for the quantitative evaluation of various compounds. Their advanced UV/Vis spectrophotometers are engineered to deliver exceptional sensitivity and accuracy, which are vital for determining the concentration of active ingredients in formulations. Notably, research indicates that laboratories utilizing PerkinElmer spectrophotometers achieve regulatory compliance more effectively, with a remarkable 95% satisfaction rate reported by users regarding their reliability in meeting control requirements.
Equipped with user-friendly interfaces and robust data analysis tools, these process analyzers streamline the analytical process, allowing researchers to focus on crucial tasks without compromising quality standards. Industry experts highlight that the integration of UV/Vis spectrophotometry in drug formulation not only bolsters compliance but also aids in the development of safer and more effective medications.
Practical applications of these spectrophotometers encompass:
- Routine quality checks
- Stability testing
This underscores their importance in upholding high-quality standards throughout the pharmaceutical industry.
Waters Corporation Chromatography Solutions: Enhancing Analysis Capabilities
Waters Corporation provides a comprehensive array of chromatography solutions that significantly elevate analytical capabilities within pharmaceutical environments. These systems are meticulously engineered for high efficiency and precision, making them exceptionally suited for complex analyses, including impurity profiling and stability testing.
The incorporation of automated sample handling not only optimizes workflows but also enhances laboratory productivity by minimizing manual intervention and reducing human error. Furthermore, integrated data management features support compliance with stringent industry regulations, ensuring that facilities can produce accurate and reliable results.
Industry leaders recognize that advancements in chromatography technology are crucial for improving efficiency in impurity profiling, ultimately facilitating the development of high-quality pharmaceuticals. Notably, statistics reveal that over 80% of novel drugs submitted to regulatory authorities have leveraged Waters' Empower Software, highlighting the essential role these systems play in bolstering laboratory efficiency and effectiveness.
Bruker Mass Spectrometry Solutions: Detailed Process Analysis
Bruker mass spectrometry solutions represent the pinnacle of analytical technology, providing profound insights into molecular structures and compositions that are vital for pharmaceutical research. These systems find extensive application in:
- Drug discovery
- Metabolomics
- Standards control
This underscores their versatility and critical importance in the industry. Equipped with high-resolution capabilities and sophisticated data evaluation software, Bruker's mass spectrometers empower researchers to identify and quantify compounds with exceptional precision. Notably, the integration of advanced mass spectrometry techniques has been demonstrated to enhance the identification of low-mass molecules, significantly streamlining drug development processes. By incorporating these innovative solutions into their operations, facilities can elevate their analytical capabilities, pushing the boundaries of drug discovery while ensuring compliance with stringent standards.
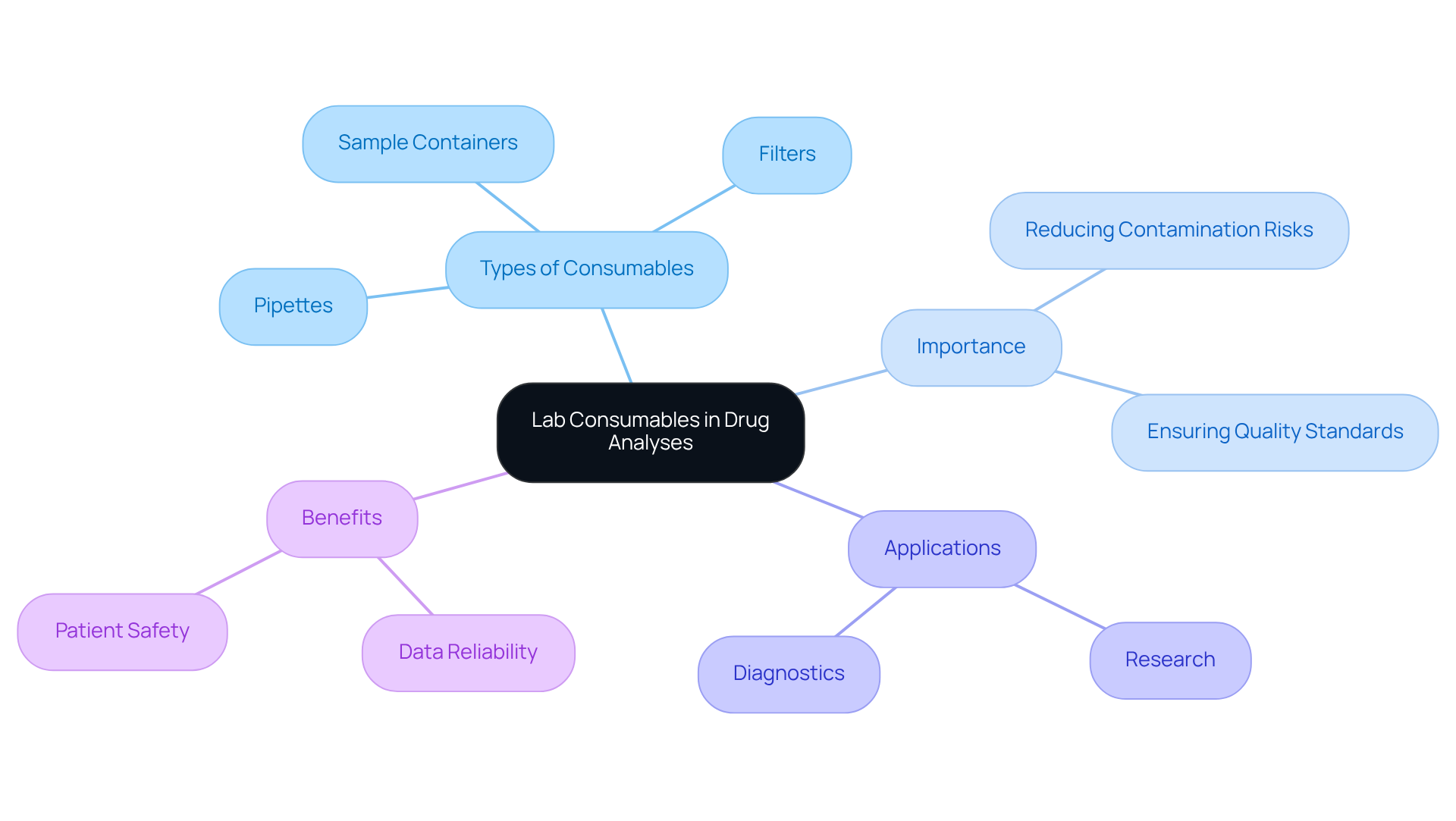
Sartorius Lab Consumables: Ensuring Efficiency in Analysis
In 2025, the significance of high-quality lab consumables in drug analyses is paramount. Products such as pipettes, filters, and sample containers are meticulously engineered to uphold the highest quality standards, ensuring that laboratories can achieve optimal results. Dependable supplies significantly reduce contamination risks, which is essential for preserving the quality of medical products. As industry leaders emphasize, effective contamination control is crucial for accurate scientific conclusions and compliance with regulatory requirements.
Real-world applications of these consumables vividly illustrate their impact on analysis efficiency. Consistent media formulations enhance cell viability and reproducibility, vital components for research and diagnostics. Moreover, the use of high-quality consumables supports robust cell populations, ultimately leading to more reliable data and improved patient safety in biomanufacturing processes.
By integrating high-quality consumables into laboratory workflows, pharmaceutical labs can streamline their operations while ensuring the integrity of their products. This unwavering commitment to quality is reflected in partnerships with leading manufacturers, further enhancing the value proposition of these essential tools within the scientific community.
Conclusion
The landscape of pharmaceutical labs is increasingly shaped by innovative process analyzers that enhance accuracy, efficiency, and compliance. This article highlights ten essential tools pivotal in ensuring the quality and safety of pharmaceutical products. From advanced HPLC systems by JM Science to cutting-edge mass spectrometry solutions from Bruker, these analyzers not only streamline workflows but also significantly contribute to the integrity of drug development processes.
Key insights reveal the importance of moisture analysis through Karl Fischer titrators, the role of chromatography systems from Agilent and Waters in achieving high-throughput results, and advancements in remote patient monitoring technology exemplified by JM Science's electronic stethoscope. Furthermore, the integration of high-quality lab consumables by Sartorius underscores the necessity of maintaining rigorous standards in pharmaceutical testing. Each of these tools plays a crucial role in addressing the evolving demands of the industry, ultimately leading to improved patient outcomes.
As the pharmaceutical sector continues to evolve, embracing these advanced process analyzers is essential. Laboratories that prioritize cutting-edge technology and reliable equipment will be better equipped to meet regulatory standards and enhance their research capabilities. The ongoing commitment to innovation and quality in pharmaceutical analysis will ensure that the industry can deliver safer and more effective therapeutics, reinforcing the significance of these essential tools in the quest for excellence in healthcare.




