Overview
The article delineates four essential titrators that significantly enhance pharmaceutical lab efficiency by improving quality control, drug formulation, stability testing, and regulatory compliance. It elaborates on how specific titration techniques and best practices—such as automation and meticulous sample preparation—serve to elevate accuracy and reliability in laboratory analyses. This ultimately contributes to the development of safer and more effective medications. By adopting these practices, laboratories can ensure that their processes not only meet but exceed industry standards, fostering a commitment to excellence in pharmaceutical development.
Introduction
Titration stands at the forefront of pharmaceutical analysis, serving as a pivotal method for ensuring the accuracy and reliability of drug formulations. As the complexity of pharmaceutical products increases, it becomes essential for laboratory professionals to understand various titration techniques and their applications. This knowledge is crucial for maintaining high standards of quality control and regulatory compliance. However, the challenge lies in selecting the right titration equipment and implementing best practices to optimize efficiency and precision. What are the critical titrators that can streamline processes and enhance the effectiveness of pharmaceutical laboratories?
Understand Titration Fundamentals and Techniques
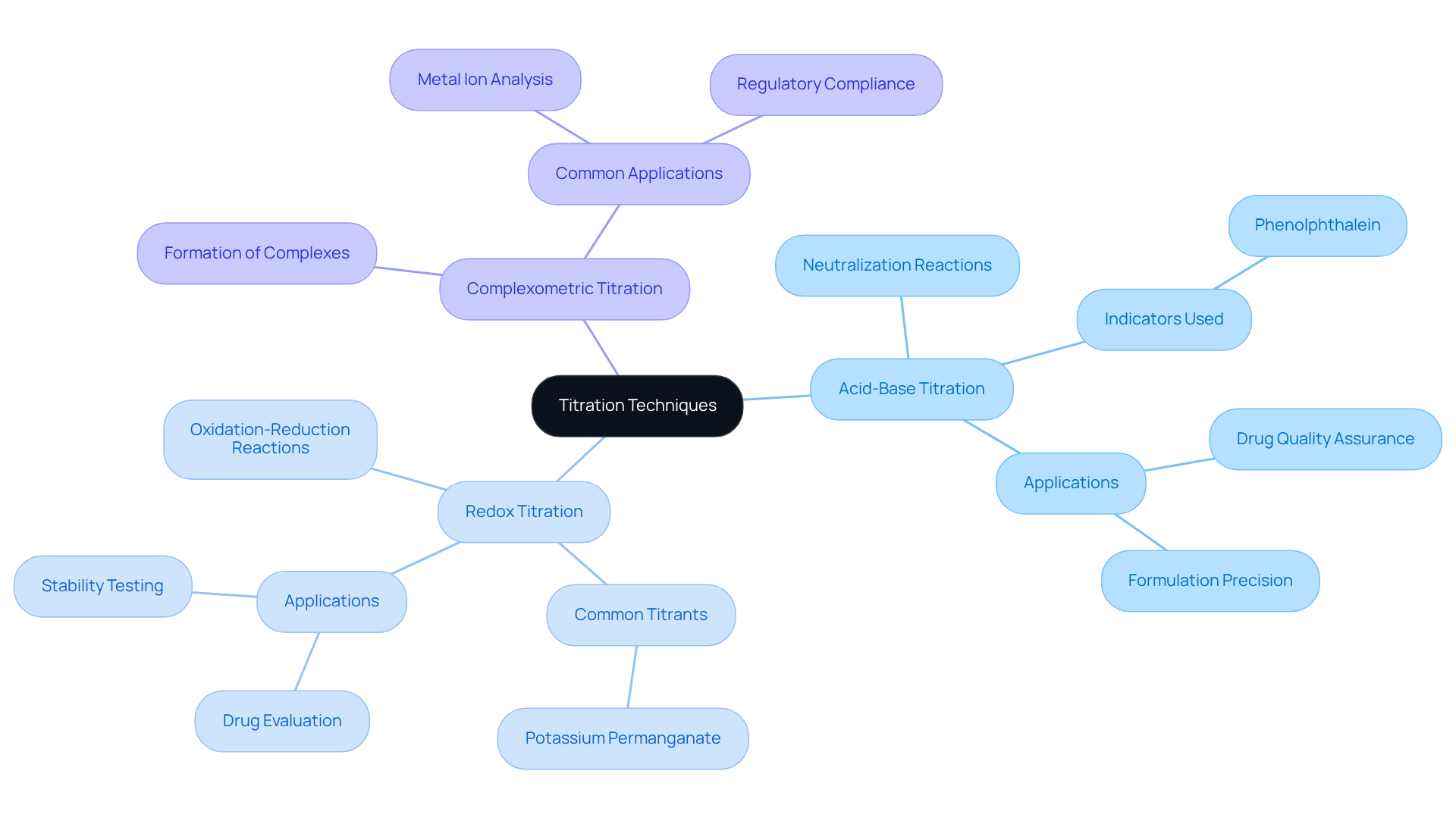
Titrators play a crucial role in titration, which is a cornerstone of quantitative analytical methods, essential for determining the concentration of unknown solutions by adding a titrant of known concentration until the reaction reaches its endpoint. Understanding the is crucial for professionals in the field, as they enable precise and effective analyses in laboratory settings. The key techniques include:
- Acid-Base Titration: This method involves a neutralization reaction between an acid and a base, commonly utilizing indicators like phenolphthalein to signal the endpoint. Acid-base analyses are essential in drug laboratories for quality assurance and formulation precision, ensuring that active components meet designated concentrations.
- Redox Titration: This technique employs oxidation-reduction reactions, often using potassium permanganate as a titrant. Redox analyses are especially important in drug evaluation, where identifying oxidizing or reducing agents is essential for determining the stability and effectiveness of medicinal products.
- Complexometric Titration: This method involves the formation of a complex between the analyte and the titrant, frequently used for metal ion analysis. Complexometric analyses are essential in the drug industry for identifying metal impurities in medication formulations, guaranteeing safety and adherence to regulatory standards.
By comprehending these techniques, laboratory staff can select titrators as the most suitable approach for their specific analytical requirements, thereby enhancing precision and effectiveness in medical applications. As chemists have observed, accuracy in measurements is not merely an objective; it is essential for significant chemistry, underscoring the importance of these techniques in maintaining high standards within drug production facilities.
Explore Titration Applications in Pharmaceutical Laboratories
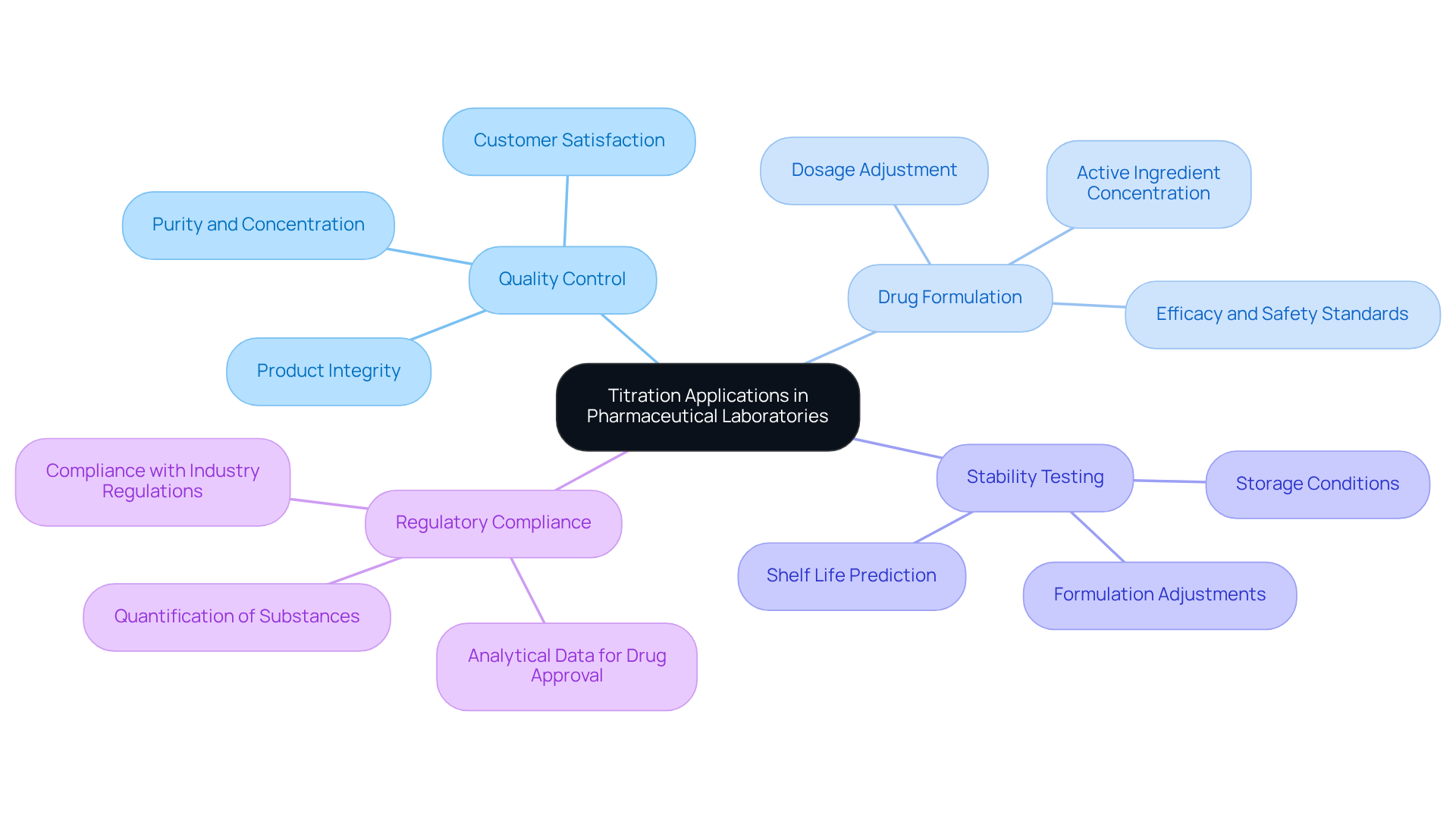
Titrators are indispensable in pharmaceutical laboratories, fulfilling multiple critical functions.
- Quality Control: Titration ensures the purity and concentration of active pharmaceutical ingredients (APIs) through precise methodologies, which are essential for maintaining product integrity and customer satisfaction. Regular analyses provide insights into how formulation changes can influence drug stability over time. JM Science Inc. offers premium titrators that improve the quality control processes, ensuring reliable results.
- Drug Formulation: This technique is vital for determining the correct concentrations of ingredients during the development of new medications. Pharmaceutical firms regularly employ dosage adjustment methods to ascertain the concentration of active components, ensuring formulations meet efficacy and safety standards. With JM Science's advanced titrators, laboratories can achieve precise measurements crucial for and HPLC solutions.
- Stability Testing: By observing the stability of formulations over time, the process evaluates changes in concentration, which is crucial for predicting shelf life and ensuring product reliability. This assessment aids in decision-making regarding storage conditions and formulation adjustments. The innovative titrators from JM Science facilitate thorough stability testing, providing the data needed for informed decisions.
- Regulatory Compliance: Titration plays a key role in meeting stringent regulatory standards by providing accurate analytical data necessary for the drug approval process. The ability to quantify the concentration of substances accurately is vital for compliance with industry regulations. JM Science's titrators are specifically designed to meet regulatory demands, ensuring that laboratories maintain compliance with confidence.
As Homar Murillo observes, "Titrations in the drug industry are primarily used for analysis, product development, and quality control." These applications emphasize the significance of mastering measurement techniques to uphold high standards in drug research and production. Nevertheless, it is essential to choose the correct indicators, as Emily White cautions, "A poor selection of indicator can make a measurement ineffective." Mastering these techniques ultimately contributes to the safety and efficacy of medications.
Implement Best Practices for Efficient Titration Processes
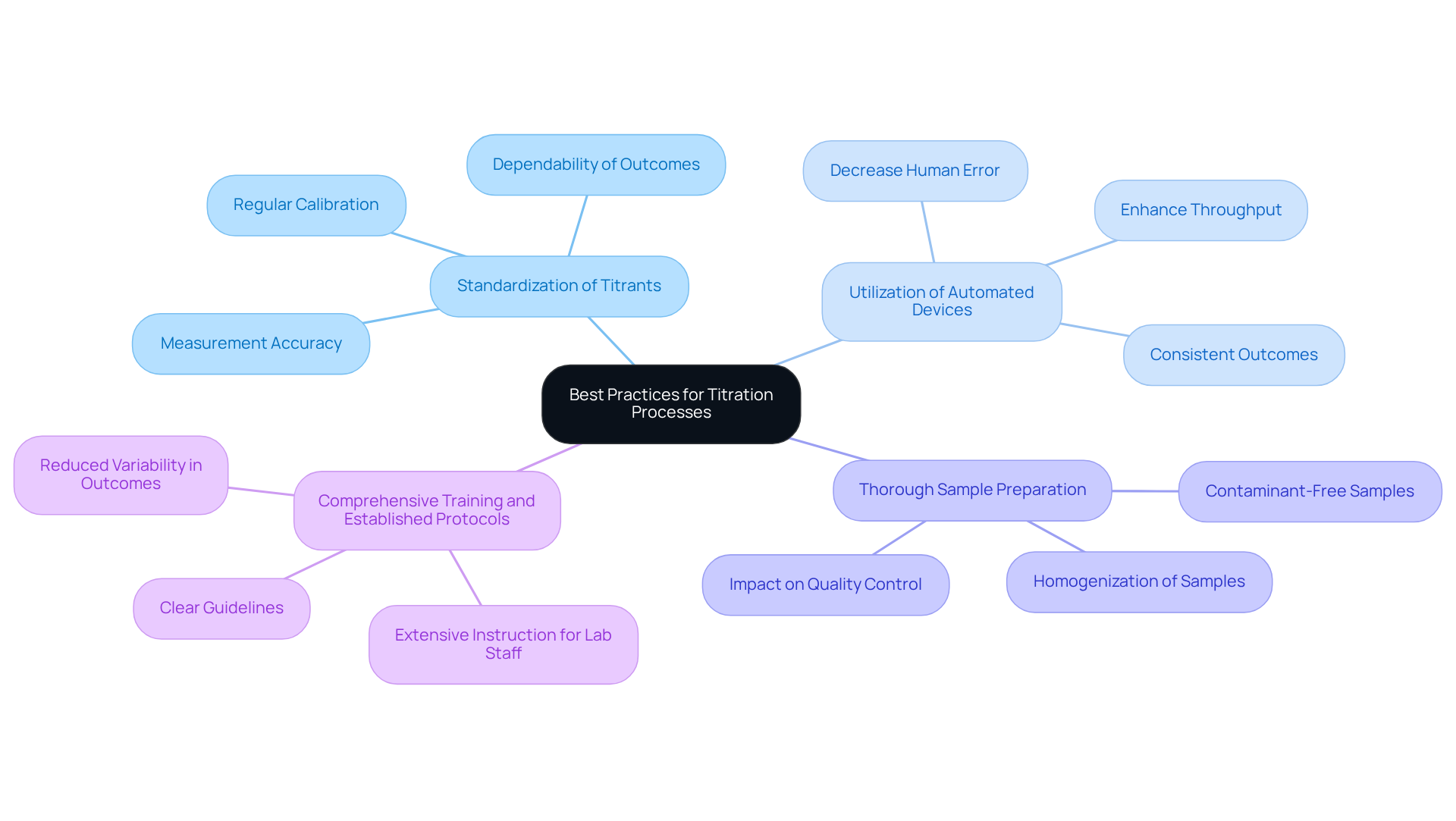
To optimize the efficiency of titration processes in pharmaceutical laboratories, it is essential to implement the following best practices:
- Standardization of Titrants: Regular calibration and standardization of titrants are crucial for ensuring measurement accuracy. This practice significantly reduces discrepancies and enhances the dependability of outcomes, which is vital in pharmaceutical applications where precision is paramount.
- : Implementing automated measuring systems markedly decreases human error and enhances throughput, particularly in high-volume laboratories. These systems optimize the measurement process, enabling consistent and repeatable outcomes that are essential for adhering to industry standards.
- Thorough Sample Preparation: Ensuring that samples are homogenized and free from contaminants is critical to avoid distorted outcomes. enhances the precision of measurement results, directly impacting quality control procedures in pharmaceutical manufacturing.
- Comprehensive Training and Established Protocols: Providing extensive instruction for lab staff on measurement techniques and creating clear guidelines can significantly reduce variability in outcomes. A well-prepared team is better equipped to manage the complexities of the process, resulting in enhanced efficiency and reliability in laboratory operations.
In addition to these practices, it is crucial to acknowledge key concepts in the process, such as the roles of titrators, titrant, analyte, endpoint, and equivalence point, which are essential for executing successful procedures. Addressing common sources of error, including human mistakes, improper indicator selection, and contamination, is necessary to mitigate challenges in the measurement process. By adhering to these best practices, pharmaceutical facilities can achieve more dependable outcomes and greatly improve overall efficiency, ultimately supporting the integrity of their analytical processes.
Select Appropriate Titration Equipment for Optimal Results
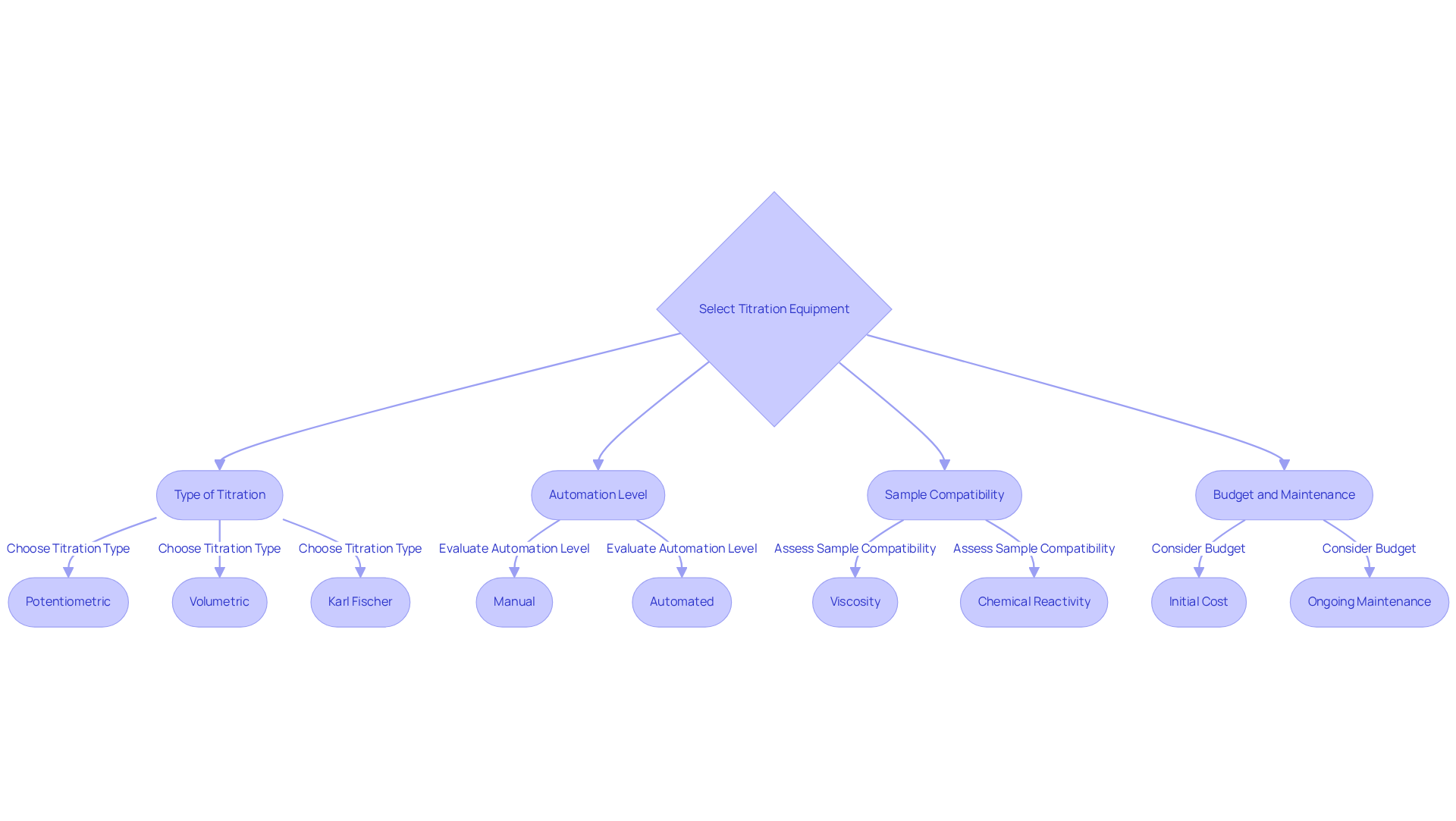
Selecting the appropriate equipment is crucial for optimizing experimental efficiency and ensuring reliable results. JM Science Inc. offers a diverse range of high-quality titrators, such as Karl Fischer titrators and potentiometric systems, designed for various measurement techniques. Furthermore, the company provides high-performance liquid chromatography (HPLC) solutions, encompassing columns and accessories vital for comprehensive laboratory analysis. Key considerations include:
- Type of Titration: It is essential to choose equipment specifically designed for the required titration method, whether potentiometric, volumetric, or Karl Fischer. Each method possesses distinct requirements and applications that must be addressed.
- Automation Level: Evaluate the suitability of manual versus in relation to your laboratory’s workload and precision needs. JM Science's automated measurement solutions are increasingly recognized for their speed and accuracy, significantly minimizing human error and enhancing throughput, particularly in high-demand settings. Dr. Veronica N. Hall aptly remarks, 'The transition to automated titrators heralds a new era where accuracy meets efficiency.'
- Compatibility with Samples: Confirm that the selected equipment is compatible with the sample types under analysis. JM Science's titrators are designed to effectively manage various sample matrices, guaranteeing precise results regardless of viscosity or chemical reactivity. Factors such as viscosity, chemical reactivity, and analyte characteristics are critical for achieving accurate results.
- Budget and Maintenance: Consider the total cost of ownership, which encompasses the initial purchase price, ongoing maintenance, and consumables. Although automated systems may entail a higher initial investment, they frequently yield long-term savings through enhanced efficiency and reduced labor costs. Shankar Godavarti emphasizes that "automated measuring devices are gaining significant traction, owing to their advantages in terms of speed, accuracy, and reduced human error."
By judiciously selecting measurement apparatus from JM Science, laboratories can elevate their analytical capabilities, streamline processes, and improve the reliability of their findings. Additionally, it is vital to recognize common pitfalls in titration method selection, such as misidentifying the analyte type or overlooking the sample matrix, as these can lead to inaccurate results.
Conclusion
Understanding the intricacies of titration is paramount for enhancing efficiency in pharmaceutical laboratories. This article underscores the essential role of titrators in various analytical processes, emphasizing their critical importance in ensuring the accuracy and reliability of drug formulations. By mastering titration techniques and selecting the appropriate equipment, laboratories can significantly elevate their operational standards and uphold the integrity of their products.
Key insights discussed include fundamental techniques of titration, such as:
- Acid-base titrations
- Redox titrations
- Complexometric titrations
Each serving specific applications in pharmaceutical settings. Furthermore, the article highlights best practices, including:
- The standardization of titrants
- Automation
- Thorough sample preparation
These collectively contribute to achieving precise measurements and compliance with regulatory standards. The careful selection of titration equipment is crucial for optimizing laboratory processes, necessitating a thoughtful consideration of factors like automation level and sample compatibility.
Ultimately, the effective implementation of titration methods transcends mere technical requirements; it is a foundational element that underpins the safety and efficacy of pharmaceutical products. By embracing the latest advancements in titration technology and adhering to best practices, pharmaceutical laboratories can enhance their analytical capabilities, ensure product quality, and meet the rigorous demands of the industry. Proactively optimizing titration processes will not only improve laboratory efficiency but also contribute significantly to the overall success of pharmaceutical research and production.




