Overview
This article delineates four essential practices for the analysis of hydrogen peroxide in laboratory environments.
- Understanding its properties is paramount, as it lays the foundation for effective sample preparation.
- Advanced analytical methods must be employed to ensure accuracy and reliability in measurements.
- Establishing stringent quality control measures is crucial for compliance with regulatory standards.
- Together, these practices not only promote safe handling but also enhance the integrity of hydrogen peroxide lab results.
By adopting these practices, laboratories can significantly improve their analytical outcomes.
Introduction
Understanding the properties and safe handling of hydrogen peroxide (H2O2) is essential in laboratory settings. Its potent oxidizing capabilities can pose significant risks if not managed properly. This article delves into four key practices that enhance the accuracy of hydrogen peroxide analysis and ensure compliance with regulatory standards. As laboratories strive for precision, the challenge remains: how can they effectively balance rigorous analytical methods with the necessity for safety and quality control?
Understand Hydrogen Peroxide Properties and Regulatory Standards
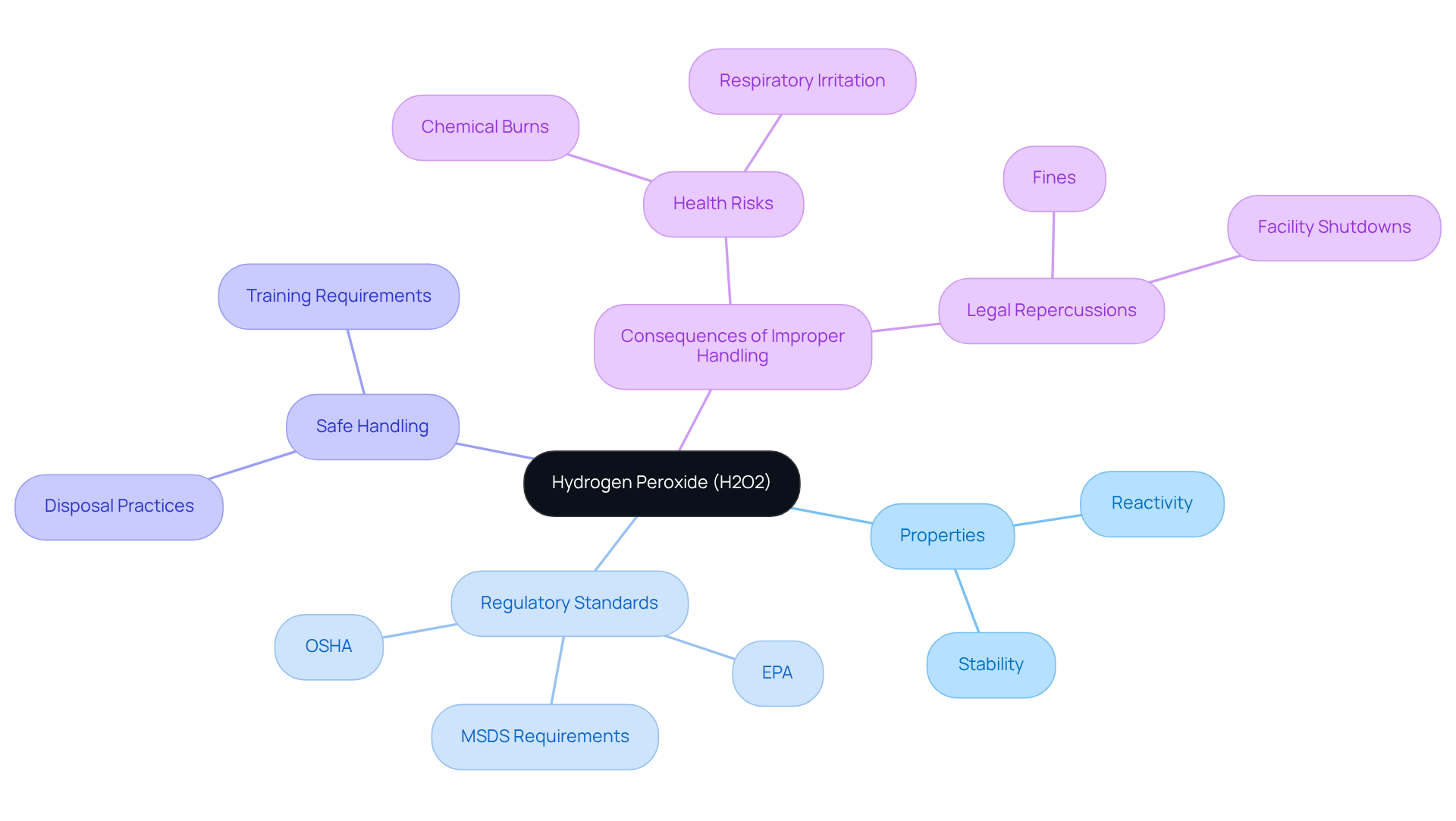
Hydrogen peroxide (H2O2) serves as a potent oxidizing agent, widely employed in various applications such as disinfection and bleaching. Its properties, including reactivity and stability, fluctuate based on concentration and environmental conditions. Understanding these characteristics is crucial for laboratory personnel to guarantee safe handling and precise analysis of hydrogen peroxide lab experiments.
Regulatory standards established by OSHA and EPA govern the safe application and disposal of H2O2, underscoring the need for compliance. Laboratories are required to maintain Material Safety Data Sheets (MSDS) for this chemical and ensure that all personnel receive training on its hazards and safe handling protocols.
Small quantities of diluted hydrogen peroxide (≤3%) may be safely disposed of down the drain, contingent upon local regulations. Adhering to these standards not only safeguards laboratory staff but also preserves the integrity of the analysis of hydrogen peroxide lab results.
Improper handling can result in serious health risks, including chemical burns and respiratory irritation, particularly at concentrations exceeding 8%. Furthermore, inadequate disposal practices can elevate oxygen levels in aquatic systems, disrupting ecosystems and posing environmental threats.
Non-compliance with OSHA and EPA regulations may lead to legal repercussions, such as fines or facility shutdowns, highlighting the critical importance of adhering to these guidelines.
Implement Effective Sample Preparation Techniques
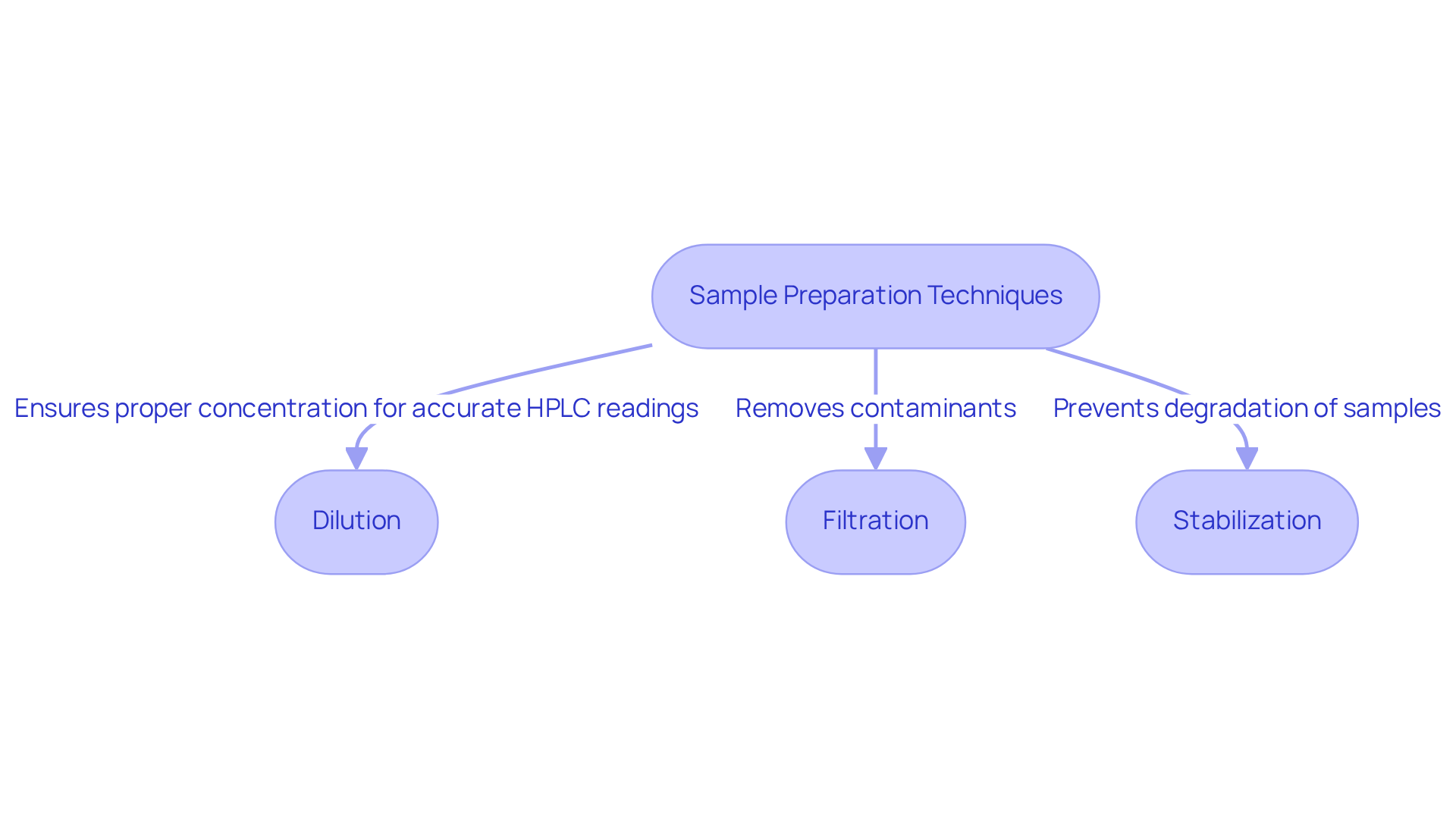
Sample preparation stands as a pivotal phase in the examination of H2O2, where methods such as:
- dilution
- filtration
- stabilization
are essential to ensure that specimens are both representative and free of contaminants. For instance, in the context of HPLC analysis, diluting oxygenated water to appropriate concentrations is critical to prevent column damage and to guarantee accurate readings. Furthermore, employing high-quality reagents and meticulously clean glassware is vital to avert cross-contamination. By establishing a standardized procedure for sample preparation, laboratories can significantly enhance the reliability of testing outcomes in the analysis of hydrogen peroxide lab, thereby facilitating the comparison of data across various experiments and studies.
Utilize Advanced Analytical Methods and Instruments
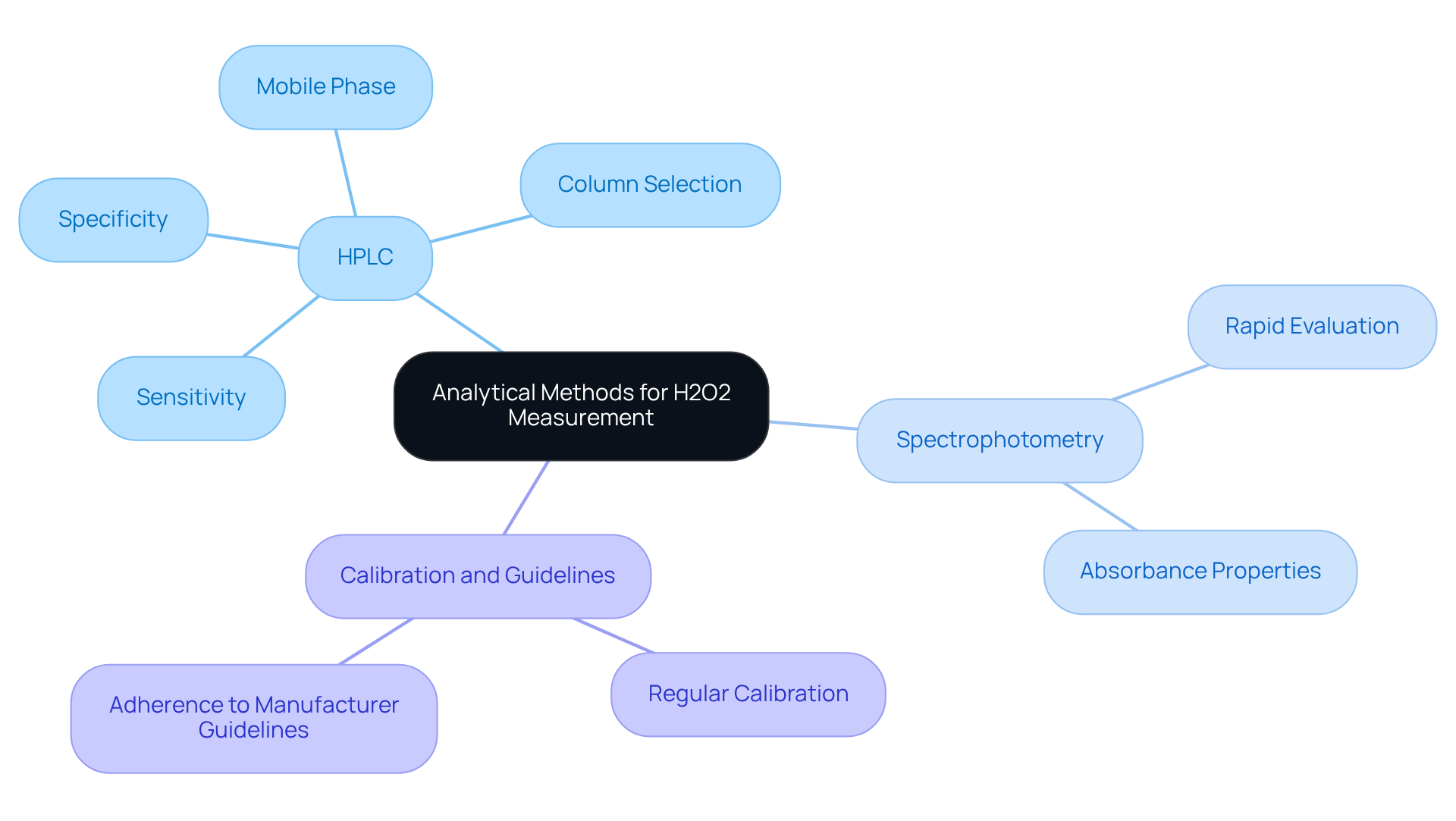
Sophisticated evaluation techniques, such as High-Performance Liquid Chromatography (HPLC) and spectrophotometry, are essential for the precise measurement of H2O2 concentrations. HPLC, in particular, provides exceptional sensitivity and specificity, making it the preferred choice for analyzing complex matrices.
Selecting the appropriate column and mobile phase is critical when utilizing HPLC, as it directly impacts the optimal separation and detection of analytes. Additionally, spectrophotometric methods offer rapid evaluations of H2O2 concentration, leveraging its unique absorbance properties.
To ensure accuracy and reliability in test results, regular calibration of instruments and strict adherence to manufacturer guidelines are imperative.
Establish Quality Control and Documentation Practices
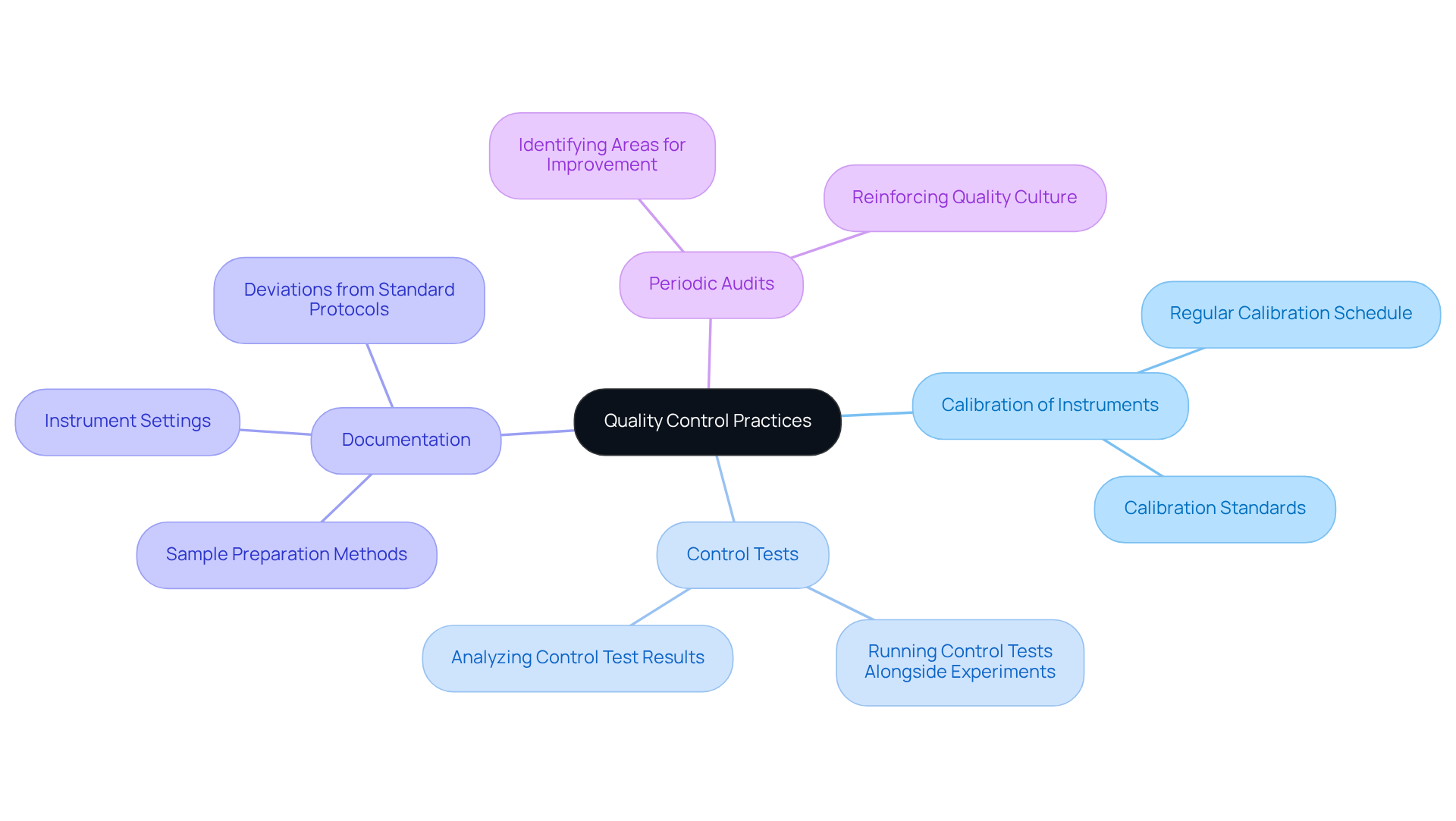
Establishing robust quality control (QC) practices is vital for the analysis of hydrogen peroxide lab. Regular calibration of analytical instruments, running control tests alongside experimental measures, and meticulous documentation of all analytical procedures are essential components. Documentation must encompass details such as sample preparation methods, instrument settings, and any deviations from standard protocols. This meticulous approach not only facilitates troubleshooting but also ensures compliance with regulatory requirements. Furthermore, periodic audits of laboratory practices can identify areas for improvement, reinforcing a culture of quality within the laboratory.
Conclusion
Understanding and implementing the key practices for the analysis of hydrogen peroxide is essential for achieving accurate and reliable laboratory results. This article underscores the critical importance of:
- Grasping the properties and regulatory standards of hydrogen peroxide
- Adopting effective sample preparation techniques
- Utilizing advanced analytical methods
- Establishing robust quality control and documentation practices
By adhering to these principles, laboratories can enhance the integrity of their analyses while ensuring the safety of personnel and compliance with legal regulations.
The discussion emphasizes that safe handling and disposal of hydrogen peroxide, in accordance with OSHA and EPA guidelines, are paramount in preventing health risks and environmental harm. Rigorous sample preparation methods, such as dilution and filtration, are crucial for maintaining the quality of test results. Advanced techniques like HPLC and spectrophotometry further contribute to precise measurements, while diligent quality control practices bolster the reliability of findings and facilitate continuous improvement within laboratory settings.
Ultimately, the significance of these practices extends beyond mere compliance; they foster a culture of safety, accuracy, and responsibility in laboratories handling hydrogen peroxide. Embracing these best practices not only protects laboratory staff and the environment but also paves the way for innovative research and development in the field. Laboratories are encouraged to prioritize these guidelines to enhance their operational standards and contribute to the broader scientific community.




