Overview
This article highlights four essential practices for effective ion analysis in pharmaceuticals:
- Selecting appropriate methods
- Optimizing sample preparation
- Ensuring regular calibration and maintenance
- Utilizing advanced detection methods
These practices are crucial for enhancing the precision and reliability of ion analysis, as they adhere to regulatory standards and leverage technological advancements. Ultimately, they contribute significantly to the safety and efficacy of pharmaceutical products, reinforcing the importance of high-quality scientific instruments in laboratory settings.
Introduction
In the intricate world of pharmaceuticals, the precision of ion analysis serves as a critical pillar for ensuring drug safety and efficacy. As the industry evolves, the methodologies and technologies that underpin this essential process are also advancing, providing laboratories with opportunities to enhance their quality assurance practices. However, with increasing regulatory scrutiny and the demand for higher standards, pharmaceutical companies face the challenge of effectively navigating the complexities of ion analysis. How can they ensure compliance while optimizing their operations?
Understand the Importance of Ion Analysis in Pharmaceuticals
Ion analysis serves as a cornerstone in the drug industry, especially for the characterization and quantification of active pharmaceutical ingredients (APIs), excipients, and impurities. This evaluation is crucial for ensuring drug purity and efficacy, especially given that many APIs are found in salt forms, necessitating precise ion analysis to confirm their quality. Regulatory bodies, including the FDA, impose stringent testing protocols to avert contamination and protect patient safety. For example, ion analysis is extensively conducted using ion chromatography (IC) to analyze counter ions and impurities, establishing itself as a preferred technique within pharmaceutical environments.
Recent advancements in ion analysis techniques, including the integration of ambient ionization mass spectrometry (AIMS), have significantly enhanced the speed and accuracy of drug quality assessments. These innovations underscore a growing trend towards immediate evaluation, empowering facilities to prioritize ion analysis in their quality assurance procedures. By embracing these practices, drug companies can ensure the production of safer and more effective medications. Ultimately, this commitment contributes to improved patient outcomes, reinforcing the indispensable role of ion analysis in the pharmaceutical landscape.
Implement Effective Ion Analysis Techniques
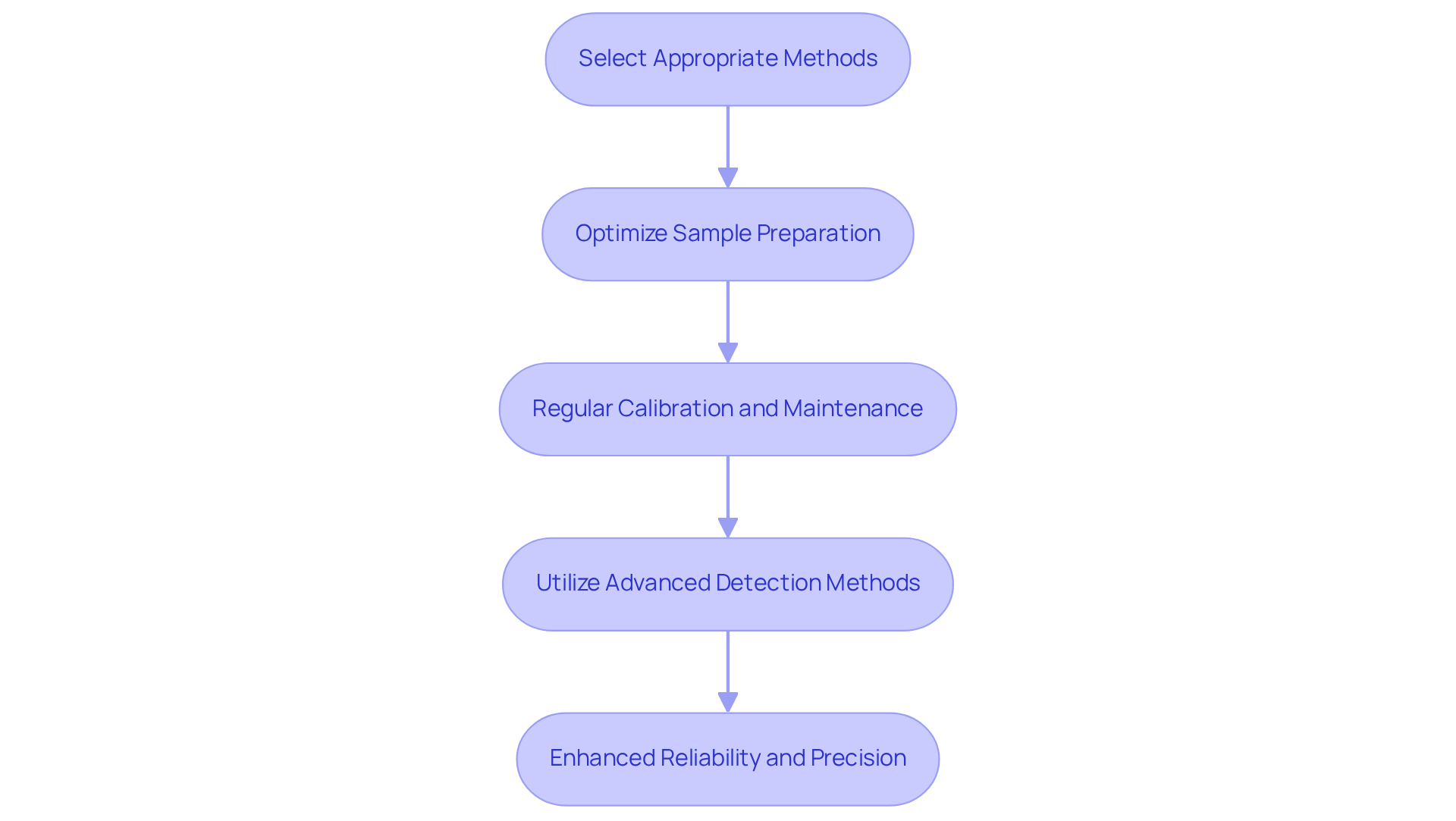
To implement effective ion analysis, laboratories must adhere to best practices that ensure precision and reliability in their results.
-
Select Appropriate Methods: Ion chromatography (IC) stands out as a robust choice for the separation and quantification of ionic species. Techniques such as suppressed ion chromatography not only enhance sensitivity but also minimize background noise, leading to more accurate analytical outcomes. The ion chromatography market is projected to grow from US$ 1.1 billion in 2024 to US$ 1.5 billion by 2031, reflecting its increasing adoption in pharmaceutical applications, with a CAGR of 8.28% from 2024 to 2032.
-
Optimize Sample Preparation: Proper sample preparation is crucial for minimizing interferences and ensuring dependable results. Laboratories should implement appropriate filtering and dilution of samples to prevent contamination. The compatibility of the column's base material with the sample matrix significantly impacts the quality of evaluation, making it imperative to select materials that avoid undesirable interactions.
-
Regular Calibration and Maintenance: Consistent calibration and maintenance of instruments in accordance with manufacturer specifications are vital for optimal equipment performance. This practice leads to consistent and accurate results. Regulatory guidelines, including those from the FDA, underscore the importance of method validation, which encompasses evaluating accuracy, precision, and specificity.
-
Utilize Advanced Detection Methods: Incorporating advanced detection techniques, such as mass spectrometry coupled with ion chromatography (IC-MS), significantly enhances the sensitivity and specificity of ion analysis. This combination allows for the identification of trace contaminants that might otherwise be overlooked, addressing critical safety concerns in drug manufacturing.
By implementing these strategies, facilities can greatly enhance the dependability and precision of their ion analysis, resulting in improved quality control in drug manufacturing.
Ensure Compliance with Regulatory Standards
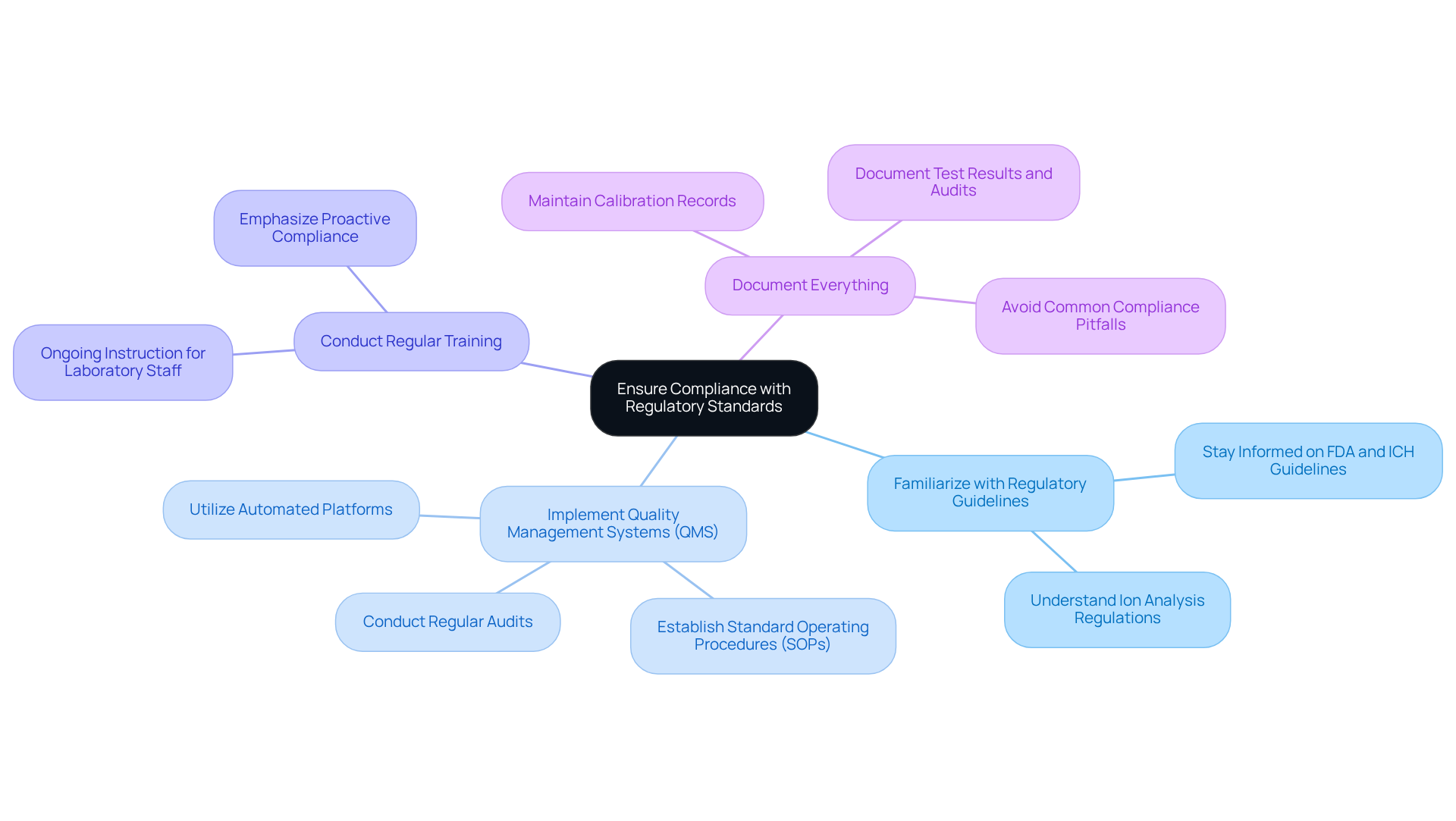
Ensuring adherence to regulatory standards is essential for pharmaceutical facilities. To achieve this, it is crucial to implement key practices that not only align with industry standards but also enhance public health and the reliability of medical products.
-
Familiarize with Regulatory Guidelines: Staying informed about the latest guidelines from regulatory bodies such as the FDA and ICH is paramount. Grasping these regulations is vital for facilities to align their practices with industry standards, particularly in ion analysis.
-
Implement Quality Management Systems (QMS): Establishing a comprehensive QMS that includes standard operating procedures (SOPs) specifically for ion assessment is essential. This system should encompass documentation, training, and regular audits to ensure ongoing compliance with FDA guidelines. Utilizing automated platforms can streamline audit processes and enhance data integrity, aligning with the evolving regulatory landscape.
-
Conduct Regular Training: Ongoing instruction for laboratory staff on regulatory requirements and best practices in ion evaluation is critical. This ensures that staff are well-informed and equipped to maintain compliance effectively. Emphasizing proactive compliance not only serves as a competitive advantage in the industry but also fosters a culture of excellence.
-
Document Everything: It is non-negotiable to maintain meticulous documentation of all processes related to ion analysis, including calibration records, test results, and compliance audits. Comprehensive documentation is essential for demonstrating compliance during inspections and audits, reinforcing the integrity of medical products. Additionally, being aware of common pitfalls in compliance procedures can assist facilities in avoiding costly errors.
By adhering to these practices, laboratories can ensure that their processes for ion analysis not only meet regulatory standards but also contribute positively to public health. As noted by industry experts, "Proactive compliance is viewed as a competitive advantage rather than a reactive burden," underscoring the importance of a forward-thinking approach.
Leverage Advanced Instrumentation for Enhanced Analysis
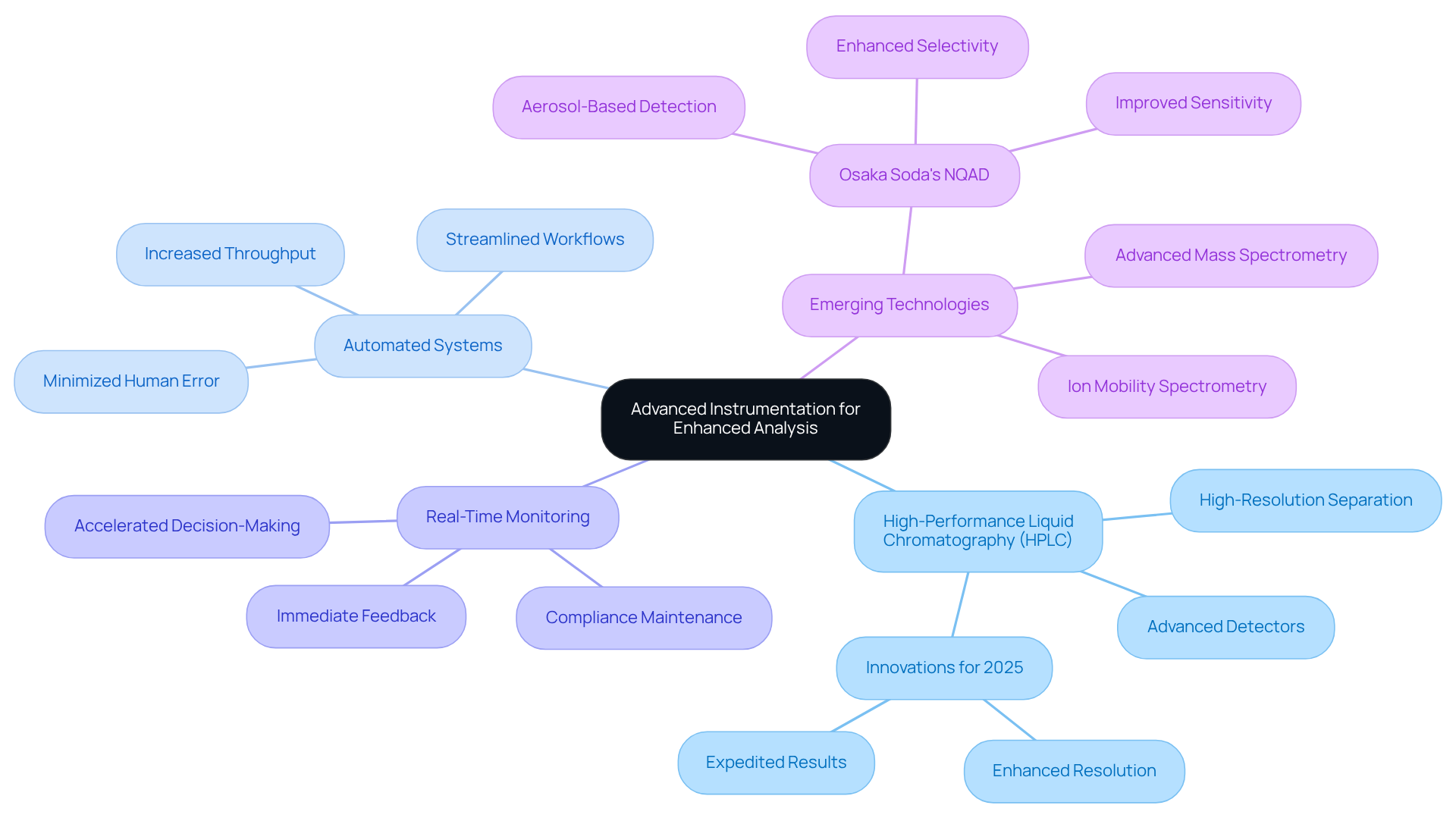
Improving ion evaluation in pharmaceuticals necessitates the utilization of advanced instrumentation. To achieve this, consider the following essential recommendations:
-
Invest in High-Performance Liquid Chromatography (HPLC): Modern HPLC systems, particularly those equipped with advanced detectors, deliver high-resolution separation of ionic species, markedly enhancing the precision of ion evaluation. Innovations in HPLC technology for 2025 emphasize enhanced resolution and expedited results, rendering them indispensable for accurate measurements.
-
Utilize Automated Systems: The implementation of automated sample preparation and evaluation systems can significantly boost throughput while minimizing human error. Automation streamlines workflows, enhances reproducibility, and enables laboratories to effectively manage larger sample volumes, which is crucial in medical environments.
-
Incorporate Real-Time Monitoring: Instruments that facilitate real-time monitoring of ion concentrations during evaluation provide immediate feedback, thereby accelerating decision-making in quality control processes. This capability is essential for maintaining compliance and ensuring product safety in pharmaceuticals.
-
Explore Emerging Technologies: Staying informed about emerging technologies, such as ion mobility spectrometry and advanced mass spectrometry techniques, can yield new insights and improve the sensitivity of evaluations. Furthermore, integrating Osaka Soda's NQAD, a groundbreaking aerosol-based detector, can significantly enhance selectivity and sensitivity in analytical applications. The NQAD employs proprietary technology to condense moisture on aerosol particles, allowing for highly sensitive detection with greater accuracy than traditional UV and MS detectors. This makes it an ideal choice for capturing elements often overlooked in previous evaluations.
By leveraging advanced instrumentation, including the NQAD, laboratories can substantially enhance their analytical capabilities, leading to more reliable and efficient processes for ion analysis tailored for pharmaceutical applications.
Conclusion
Ion analysis is a critical component in the pharmaceutical industry, serving as the backbone of medication quality and safety. Its essential role in the characterization and quantification of active pharmaceutical ingredients (APIs) and impurities is paramount. By implementing effective ion analysis practices, pharmaceutical companies not only comply with stringent regulatory standards but also elevate their quality assurance processes, ultimately resulting in safer and more effective drugs.
This article highlights several key strategies for executing effective ion analysis. These include:
- Selecting appropriate methods such as ion chromatography
- Optimizing sample preparation
- Ensuring consistent calibration and maintenance
- Employing advanced detection techniques
Each of these practices significantly enhances the reliability and precision of ion analysis, which is vital for meeting regulatory requirements and protecting public health.
In an ever-evolving pharmaceutical landscape, the adoption of advanced instrumentation and adherence to regulatory guidelines are crucial for laboratories. By cultivating a culture of proactive compliance and continuous improvement, pharmaceutical facilities can enhance operational efficiency and positively impact patient outcomes. The dedication to excellence in ion analysis transcends regulatory obligations; it is a fundamental aspect of delivering high-quality healthcare solutions.




