Overview
The article delineates four essential steps for conducting effective titration analysis in a laboratory environment. These steps encompass:
- Understanding the fundamentals of titration
- Assembling the necessary equipment and reagents
- Executing the titration process
- Troubleshooting common issues
Each step is backed by comprehensive explanations of procedures, required materials, and solutions to potential challenges, underscoring the significance of precision and accuracy in achieving reliable scientific results. By following these guidelines, laboratory personnel can enhance their analytical capabilities and ensure the integrity of their findings.
Introduction
Titration analysis stands as a cornerstone of quantitative chemistry, vital for accurately determining solute concentrations in various solutions. This technique not only enhances laboratory precision but also empowers professionals to make informed decisions across multiple fields, from pharmaceuticals to environmental science. However, navigating the intricacies of titration can pose challenges. This leads many to ponder: what are the essential steps to ensure success in this critical analytical process?
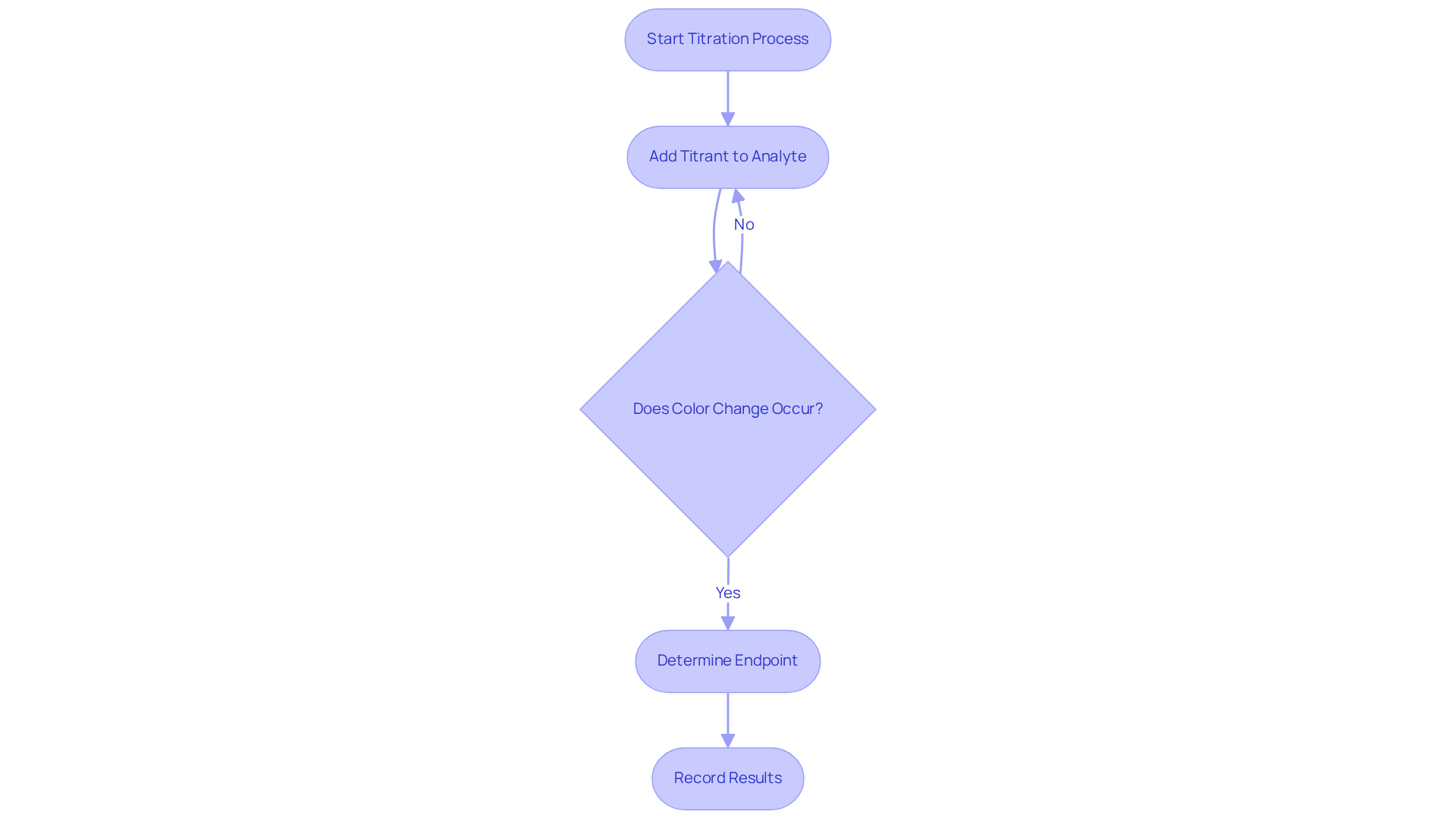
Understand the Basics of Titration
Titration is a quantitative analytical technique essential for determining the concentration of a solute in a solvent. This process involves the gradual addition of a titrant—a liquid of known concentration—to a mixture containing the analyte until the reaction reaches its endpoint. The endpoint is often indicated by a color change or a measurable alteration in a property of the mixture. Understanding the following key terms is crucial:
- Titrant: The solution of known concentration used to titrate the analyte.
- Analyte: The substance whose amount you are measuring.
- Endpoint: The point at which the reaction is complete, typically marked by a color change.
Precision in conducting titration analysis is paramount. Accurate measurements are foundational to reliable scientific outcomes. As chemists emphasize, "the key to scientific discovery is the ability to recognize and address errors." Inaccuracies can arise from various factors, including improper reagent storage and environmental exposure, which can significantly skew results. For instance, studies have shown that human error, such as misinterpreting color changes or overshooting the endpoint, can lead to erroneous estimations of concentrations, impacting laboratory results and subsequent applications.
Real-world applications of titration analysis extend across various laboratory settings, including pharmaceutical quality control and environmental monitoring. The capability to achieve precise and accurate measurements supports informed decision-making in these fields, reinforcing the necessity of rigorous training and adherence to best practices in titration procedures.
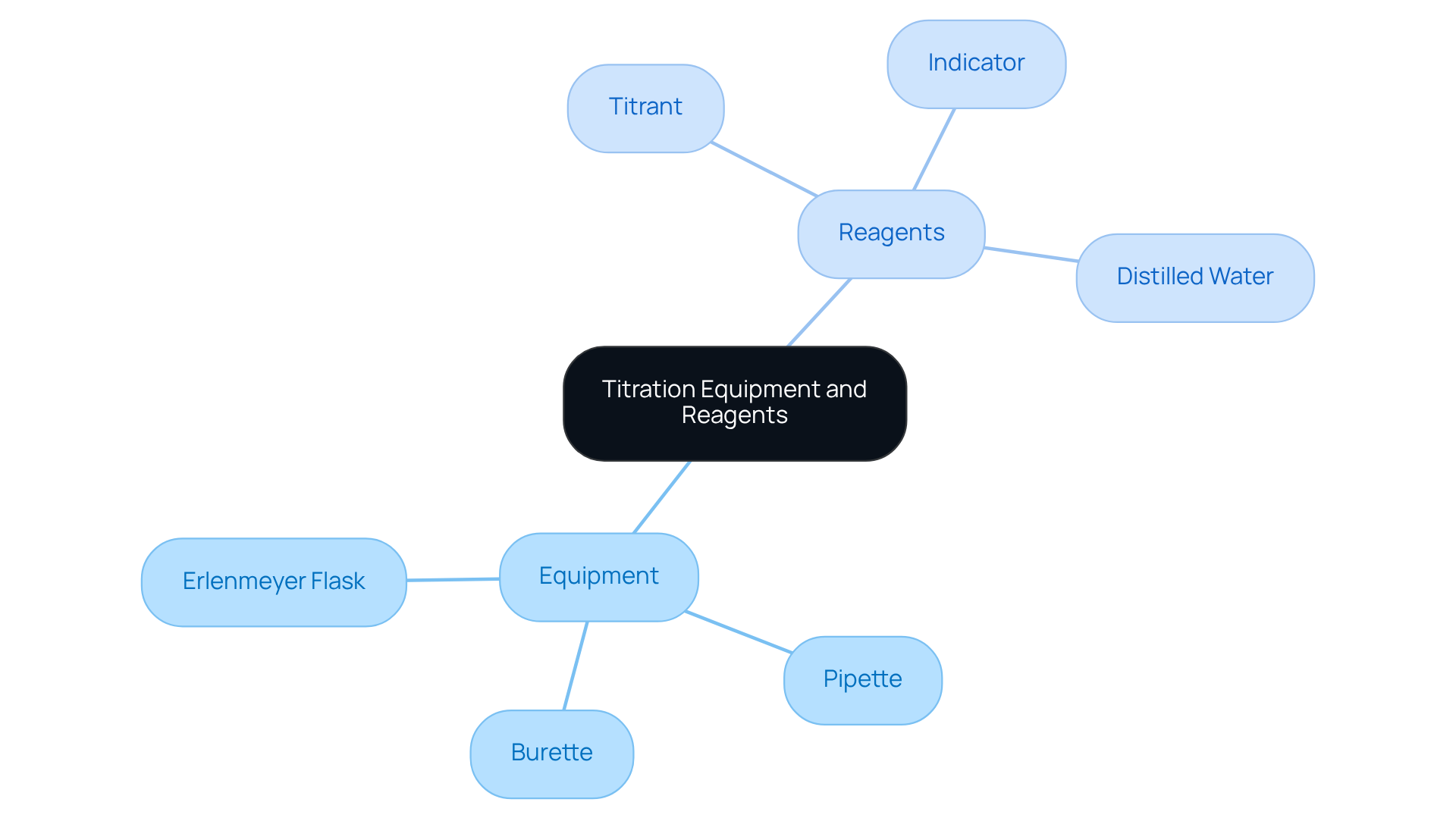
Gather Required Equipment and Reagents
Before initiating the titration process, it is imperative to have the following equipment and reagents at your disposal:
- Burette: A graduated glass tube equipped with a tap at one end, utilized for the precise delivery of the titrant.
- Pipette: Essential for accurately measuring and transferring the test liquid.
- Erlenmeyer Flask: Designed to hold the analyte liquid throughout the titration.
- Titrant: A liquid of known concentration, such as sodium hydroxide for acid-base titrations.
- Indicator: A chemical that undergoes a color change at the endpoint, with phenolphthalein being a common example.
- Distilled Water: Necessary for rinsing equipment and diluting mixtures as required.
Ensure that all equipment is meticulously cleaned and calibrated to guarantee accurate measurements. This preparation is crucial for achieving reliable results in your titration analysis experiments.
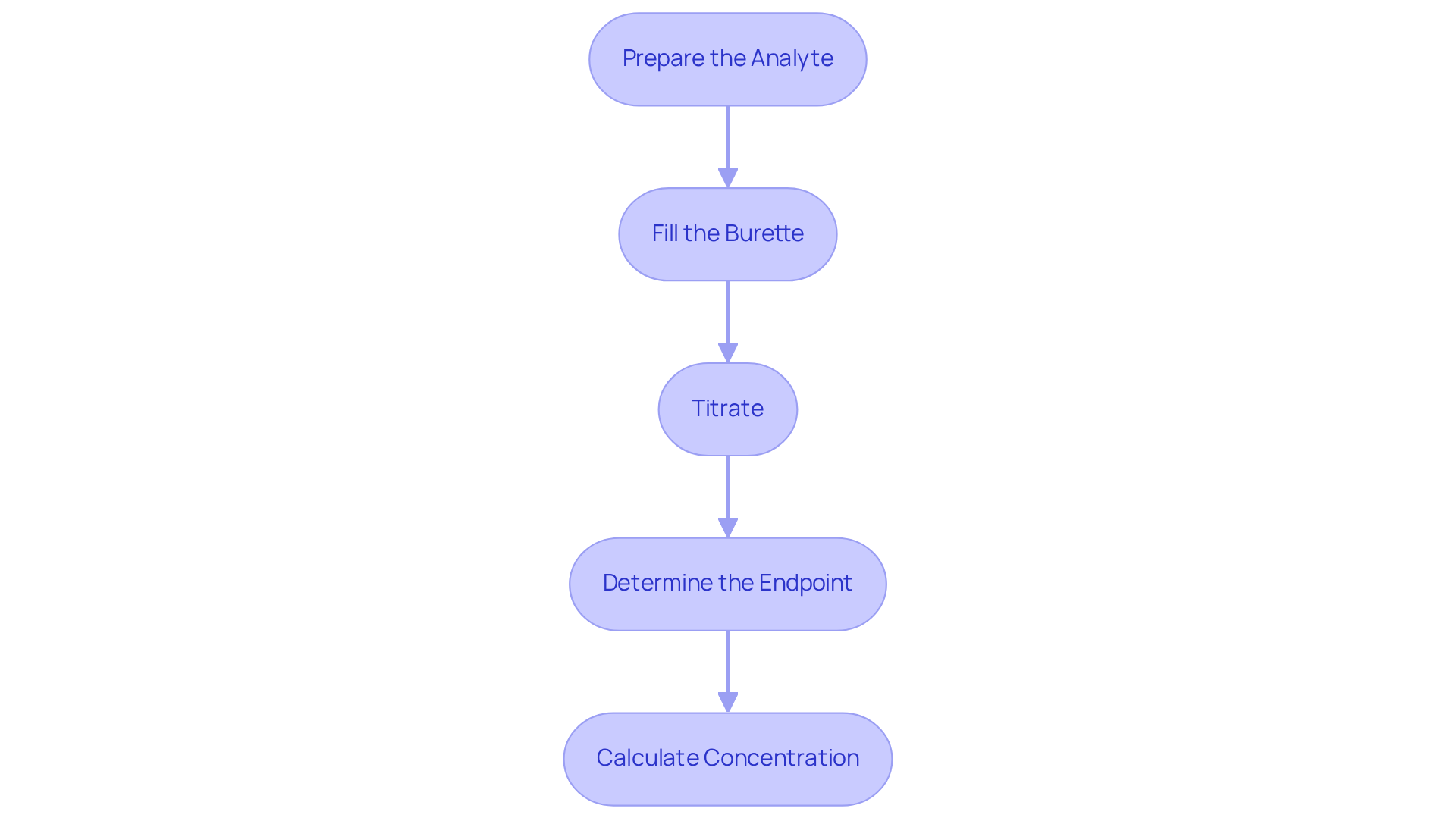
Execute the Titration Process
To perform the titration effectively, follow these essential steps:
-
Prepare the Analyte: Begin by using a pipette to measure a specific volume of the analyte liquid, transferring it into the Erlenmeyer flask. Incorporate a few drops of the chosen indicator to facilitate observation of the reaction.
-
Fill the Burette: Rinse the burette thoroughly with distilled water, followed by a rinse with the solution designated for titration. Fill the burette with the titration solution, ensuring that no air bubbles are present in the nozzle. Record the initial volume accurately.
-
Titrate: Gradually add the reagent to the analyte while continuously swirling the flask. Monitor the solution for a color change, which signifies that you are approaching the endpoint of the titration.
-
Determine the Endpoint: As you near the endpoint, add the solution dropwise until the color change becomes persistent. Document the final volume of the reagent in the burette for accurate calculations.
-
Calculate Concentration: Utilize the volume of titrant used along with its concentration to determine the concentration of the analyte. Apply the formula:
C_1V_1 = C_2V_2
where C_1 and V_1 represent the concentration and volume of the titrant, while C_2 and V_2 signify the concentration and volume of the analyte. This systematic approach ensures accurate and reliable results in your titration analysis process.
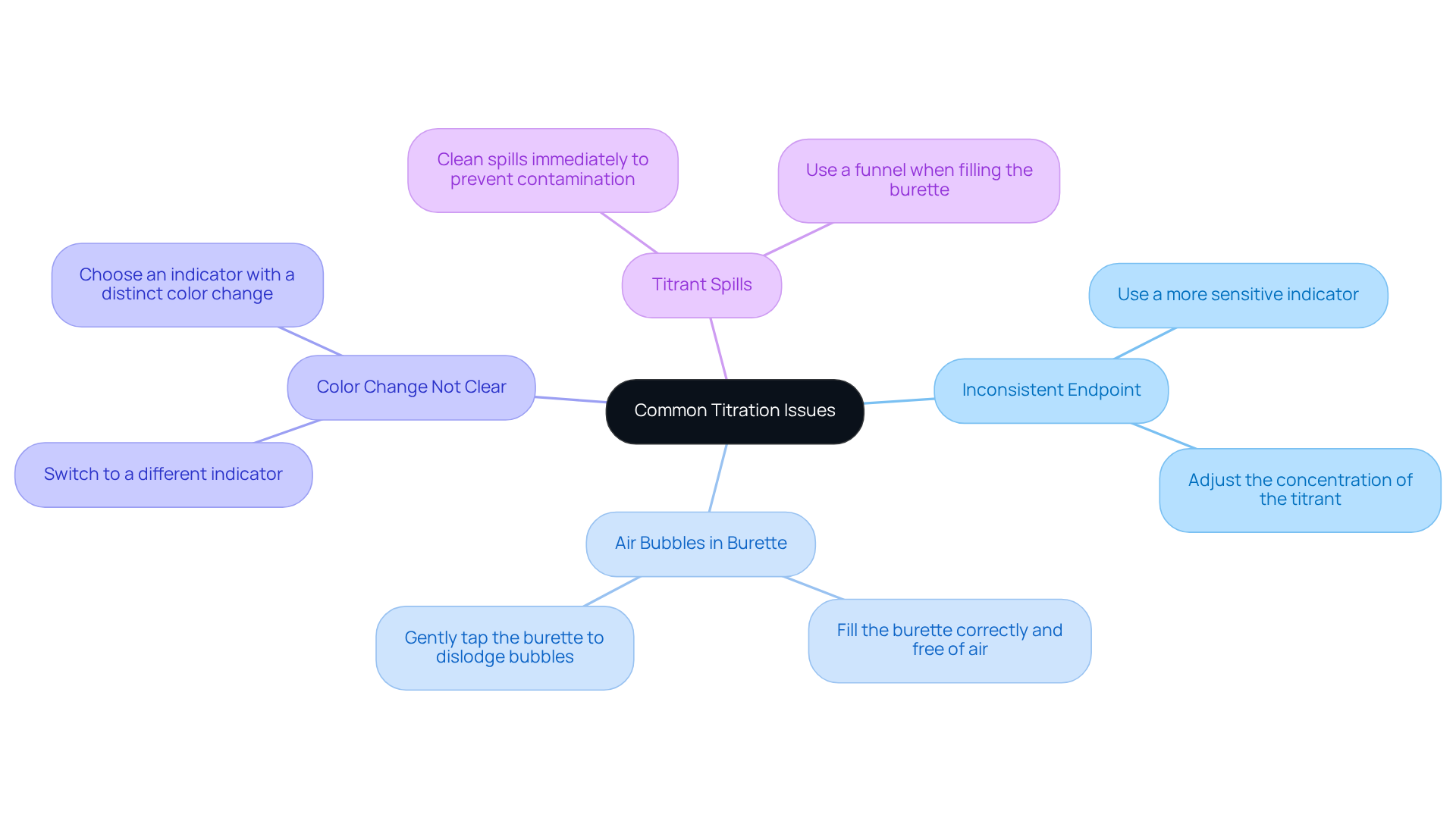
Troubleshoot Common Titration Issues
Titration analysis, which is a fundamental technique in analytical chemistry, can present several challenges. Addressing these common issues not only enhances your results but also establishes your credibility in the lab.
-
Inconsistent Endpoint: Determining the endpoint can sometimes be tricky. If you find it difficult to identify, consider employing a more sensitive indicator or adjusting the concentration of your titrant. This adjustment can lead to more reliable outcomes.
-
Air Bubbles in Burette: A common hindrance during titration is the presence of air bubbles in the burette. To prevent this, ensure that the burette is filled correctly and free of air before you begin. If you notice any bubbles, gently tap the burette to dislodge them.
-
Color Change Not Clear: Sometimes, the color change at the endpoint may be subtle. In such cases, switching to a different indicator that provides a more distinct color change can significantly improve clarity.
-
Titrant Spills: Spills can compromise your results. If they occur, it is crucial to clean them up immediately to prevent contamination and ensure accurate measurements. Always exercise caution and utilize a funnel when filling the burette to minimize this risk.
By being proactive about these potential issues and implementing the suggested solutions, you can significantly enhance the reliability of your titration analysis results.
Conclusion
Titration analysis stands as a crucial technique in analytical chemistry, enabling the precise determination of solute concentrations. Mastery of this process not only enhances experimental accuracy but also underpins the integrity of results across various applications, from pharmaceuticals to environmental monitoring. By grasping the fundamentals, assembling the necessary equipment, executing the process methodically, and troubleshooting common issues, one can ensure reliable outcomes in titration analysis.
Key arguments explored in this article underscore the importance of proper preparation and execution in titration, alongside the necessity of addressing potential pitfalls that may lead to inaccuracies. From defining essential terms such as titrant and analyte to detailing the step-by-step procedure and common troubleshooting strategies, each aspect contributes to a comprehensive understanding of effective titration practices. This structured approach is vital for achieving the desired precision and accuracy in laboratory results.
Ultimately, the significance of effective titration analysis transcends mere academic exercise; it serves as a cornerstone of scientific inquiry and quality assurance in laboratory settings. By adhering to best practices and continuously refining their skills, chemists can enhance the reliability of their analyses and contribute to informed decision-making in their respective fields. Embracing these principles not only bolsters personal expertise but also elevates the standards of scientific research as a whole.




