Overview
The title '5 Guard Strategies for Pharmaceutical Lab Managers' highlights crucial strategies that lab managers in the pharmaceutical sector must implement to optimize their operations. While the article does not delineate specific strategies, it underscores the necessity of:
- Advanced analytical instruments
- Stringent quality control measures
- Comprehensive staff training
- Integration of innovative technologies
These elements are vital for enhancing efficiency and ensuring regulatory compliance within pharmaceutical laboratories. By focusing on these critical components, lab managers can significantly improve their operational effectiveness and maintain high standards in their practices.
Introduction
In the rapidly evolving landscape of pharmaceutical research and healthcare delivery, the integration of advanced technologies is not merely an option; it is a necessity. Consider electronic stethoscopes that enhance remote patient monitoring and precise Karl Fischer titrators for moisture analysis—these innovative tools are fundamentally transforming laboratory operations and ensuring compliance with stringent regulatory standards.
As the demand for efficient and effective pharmaceutical solutions continues to rise, lab managers must prioritize:
- Quality control measures
- Staff training
- The adoption of cutting-edge analytical instruments
This article delves into the critical strategies and technologies that are shaping the future of pharmaceutical laboratories, empowering teams to improve patient outcomes and uphold high-quality standards in an increasingly competitive environment.
JM Science Electronic Stethoscope: Enhance Remote Patient Monitoring
The JM Science electronic stethoscope is meticulously engineered to enhance remote patient monitoring, empowering healthcare providers to record, replay, and transmit auscultation data seamlessly. This advanced functionality is particularly advantageous in telehealth environments, allowing clinicians to evaluate patients without the need for in-person visits. Equipped with state-of-the-art features such as noise filtering and efficient data transmission, the stethoscope guarantees high-quality sound capture, which is vital for accurate diagnoses.
As the adoption of remote patient monitoring technology has surged—evidenced by 50% of hospitals in the U.S. implementing some form of this technology by 2023—the electronic stethoscope emerges as an indispensable tool for laboratory managers. By leveraging this innovative device, they can significantly enhance patient monitoring, leading to improved health outcomes and more streamlined healthcare delivery. Furthermore, healthcare professionals have observed that such technologies not only foster better patient engagement but also elevate the overall effectiveness of telehealth services.
According to Insider Intelligence, the remote patient monitoring systems market is projected to grow by 128% by 2027, underscoring the increasing significance of these technologies. Additionally, JM Science's strategic emphasis on customer support bolsters the adoption of their electronic stethoscope, reflecting the company's unwavering commitment to innovation and customer satisfaction. This integration of advanced instrumentation, alongside JM Science's premium scientific products such as HPLC solutions and titrators, is essential for contemporary healthcare practices, particularly for lab managers in the drug industry aiming to elevate patient care.
Karl Fischer Titrators: Ensure Accurate Moisture Analysis
Karl Fischer titrators, including the AQ-300 Coulometric and AQV-300 Volumetric models from JM Science, are indispensable instruments for accurately measuring moisture content in medicinal products. Renowned for their precision, these instruments can detect moisture levels across a variety of sample types—solids, liquids, and gases—and comply with the Japanese Pharmacopoeia standards. In the drug manufacturing sector, where stringent standards are paramount, the application of Karl Fischer titration is crucial for ensuring product stability and effectiveness.
Recent advancements indicate that these titrators can achieve accuracy rates exceeding 99%, establishing them as reliable options for control standards. To sustain this level of precision, regular calibration and maintenance are essential, underscoring the importance of rigorous control protocols in drug manufacturing. JM Science offers extensive support resources, including how-to videos and application libraries, which assist lab managers in optimizing the use of these instruments.
Moreover, successful moisture analysis using Karl Fischer titration has been validated in numerous case studies, including the use of the Vapor Sorption Analyzer. This analyzer complements titration by generating high-resolution moisture sorption isotherms in under 48 hours, allowing for over 200 data points per isotherm. The synergy of both tools significantly enhances the efficiency of moisture analysis and contributes to more accurate shelf-life predictions.
As the biologics market is projected to grow at a compound annual growth rate of 15% until 2027, the demand for innovative moisture analysis solutions is set to rise. Experts in medication control emphasize that precise moisture assessment is vital for preventing stability issues that could jeopardize drug safety and efficacy. Consequently, Karl Fischer titrators remain a cornerstone of moisture analysis in the pharmaceutical sector, ensuring that products adhere to the highest standards. As Charles Eames aptly stated, "Eventually everything connects — people, ideas, objects. The standard of the connections is essential for excellence itself." To maximize the efficacy of Karl Fischer titrators, lab managers should ensure regular training and access to support resources, fostering a culture of excellence and precision in moisture analysis.
Implement Quality Control Measures: Maintain Regulatory Compliance
Establishing strict control procedures is essential for laboratory managers in the drug industry to maintain regulatory compliance and guarantee product safety. This involves regular audits, strict adherence to Good Manufacturing Practices (GMP), and meticulous documentation of all processes. By creating a culture of excellence within the laboratory, managers promote accountability and ensure that all products undergo consistent testing and verification against regulatory standards.
Moreover, the integration of automated data collection systems can significantly streamline these processes, minimizing the risk of human error and enhancing overall laboratory efficiency. Early adopters of AI-enhanced continuous improvement (CI) practices have reported reductions in development timelines by nearly 45% in certain therapeutic areas, underscoring the potential for innovation acceleration in drug laboratories. This aligns with a recent case analysis demonstrating how AI can revolutionize control processes, resulting in more efficient operations.
Furthermore, with 74% of all breaches linked to human factors, strengthening control measures becomes even more essential in protecting against compliance failures. Specific strategies, such as enhanced training and robust documentation practices, can effectively mitigate these risks. Notably, as reported by PwC, three out of five corporate risk and compliance professionals express confidence in their ability to manage compliance risks, emphasizing the significance of a proactive stance on quality control.
By prioritizing these strategies and remaining informed on GMP updates for 2025, lab managers can not only meet regulatory requirements but also contribute to the advancement of safe and effective medical products.
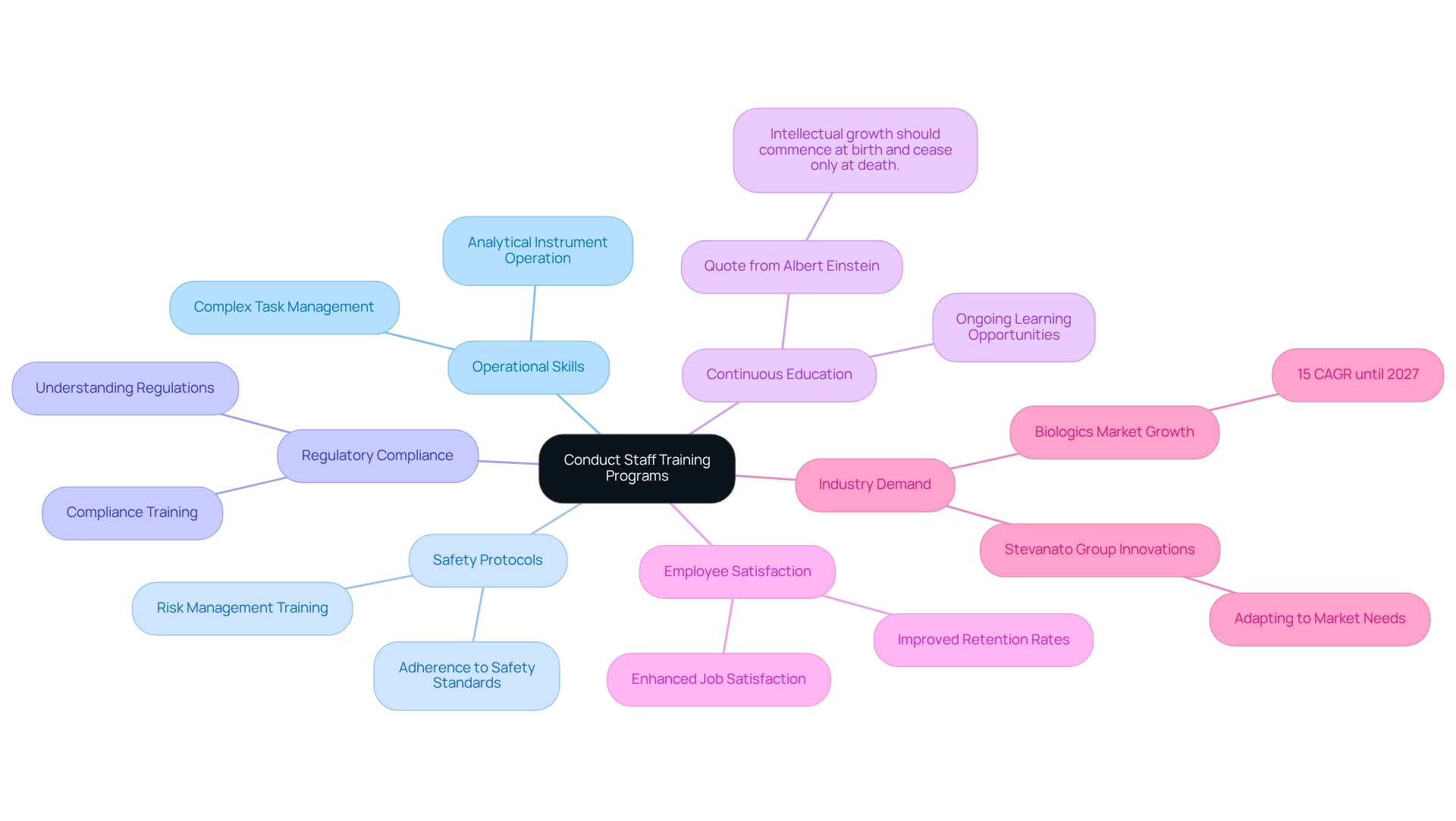
Conduct Staff Training Programs: Improve Team Efficiency
Regular employee training programs are essential for enhancing efficiency in pharmaceutical settings. These programs must encompass the operation of analytical instruments, adherence to safety protocols, and a thorough understanding of regulatory compliance. By prioritizing training, lab managers empower their teams to tackle complex tasks and seamlessly adapt to emerging technologies. As Albert Einstein wisely remarked, "Intellectual growth should begin at birth and stop only at death," underscoring the significance of ongoing education in the research environment.
Moreover, fostering an environment of continuous education not only improves employee satisfaction and retention but also significantly supports the overall productivity of the facility. With the biologics market projected to grow at a compound annual growth rate of 15% until 2027, the demand for skilled personnel is more pressing than ever. For instance, the Stevanato Group is adapting to this growth by developing innovative solutions that meet the evolving needs of the biologics sector, demonstrating how training and innovation can align with industry demands.
Investing in comprehensive training programs can lead to improved efficiency and productivity in facilities, positioning teams to meet evolving industry demands effectively. It is crucial to recognize that achievement in the experimental environment requires both preparation and continuous effort, as these elements distinguish winners from dreamers. By implementing robust training programs, drug companies can ensure their teams are well-prepared to navigate the complexities of their work.
Adopt Advanced Analytical Instruments: Streamline Research Processes
Adopt Advanced Analytical Instruments: Streamline Research Processes
The adoption of advanced analytical instruments is crucial for pharmaceutical lab managers seeking to optimize their research processes. High-performance liquid chromatography (HPLC) and mass spectrometers are particularly noteworthy for their exceptional sensitivity and specificity, enabling comprehensive sample analysis. By integrating these technologies, laboratories can substantially decrease analysis times while improving the reliability of their results.
Recent trends reveal that the clinical biomarker testing market is anticipated to grow from USD 873.6 million in 2023 to USD 1,750.9 million by 2033, reflecting a robust annual growth rate of 7.2%. This growth underscores the increasing reliance on advanced analytical methods in drug research.
Furthermore, North America accounted for 53% of the analytical testing market in 2023, while the Asia-Pacific region is emerging as the fastest-growing area due to escalating healthcare investments and a rising demand for analytical testing services. Prominent companies such as Pfizer and Roche are partnering with Contract Research Organizations (CROs) for testing services, emphasizing the industry's dependence on advanced analytical instruments. This dynamic landscape highlights the essential role of HPLC, mass spectrometry, and innovative solutions like Osaka Soda's NQAD in enhancing research efficiency and complying with regulatory standards.
As research facilities strive to maintain a competitive edge, it is imperative to stay updated on the latest advancements in HPLC technology and aerosol-based detection. Innovations in these areas not only boost analytical capabilities but also position laboratories as leaders in research and development within the healthcare sector. The NQAD, featuring proprietary technology, provides enhanced selectivity and sensitivity compared to traditional detectors, ensuring that previously overlooked components are accurately captured. As Amgen articulates, "sophisticated testing methods are essential to meet regulatory standards and ensure the safety and efficacy of their products." By leveraging these advanced instruments, including JM Science's premium scientific tools such as HPLC columns and titrators, pharmaceutical labs can meet the evolving demands of the industry while contributing to significant advancements in healthcare.
Conclusion
The integration of advanced technologies in pharmaceutical laboratories is vital for improving patient outcomes and maintaining regulatory compliance. The JM Science electronic stethoscope exemplifies how innovative tools can enhance remote patient monitoring, enabling healthcare providers to deliver effective care without the necessity of in-person visits. As the remote patient monitoring market continues to expand, the significance of such technologies becomes increasingly evident.
Karl Fischer titrators are indispensable in moisture analysis, which is crucial for ensuring product stability and efficacy. These instruments offer high accuracy and adherence to quality standards, assisting lab managers in maintaining product integrity. Furthermore, regular training and robust quality control practices are essential for pharmaceutical companies to meet the escalating demands of the biologics market while ensuring drug safety.
Emphasizing staff training and adopting state-of-the-art analytical instruments also boosts laboratory efficiency and cultivates a culture of quality. By fostering continuous learning, teams can adeptly adapt to emerging technologies, driving productivity and innovation.
In conclusion, the future of pharmaceutical laboratories hinges on the strategic integration of advanced technologies, stringent quality control, and ongoing staff development. By prioritizing these elements, lab managers can effectively navigate industry challenges and contribute to the advancement of safe and effective healthcare solutions, ensuring that patient needs remain paramount.




