Overview
The article presents critical insights into filter sterilization syringe applications, underscoring their vital role in laboratory environments for effective sterilization and sample preparation. These syringes, particularly those featuring a 0.45 micron pore size, are indispensable across various sectors, including pharmaceuticals and biotechnology. Their capacity to efficiently eliminate contaminants is crucial for maintaining sample integrity, thereby supporting rigorous quality control and ensuring compliance with regulatory standards. In summary, the importance of high-quality scientific instruments like filter sterilization syringes cannot be overstated, as they are fundamental to achieving excellence in laboratory practices.
Introduction
In the realm of laboratory research, the landscape is increasingly reliant on precision and sterility, rendering filter sterilization syringes indispensable tools in this environment. These innovative devices not only ensure the elimination of impurities from samples but also cater to a wide array of applications across various industries, from pharmaceuticals to food safety.
As the demand for high-quality filtration solutions escalates, a pressing challenge arises: how can laboratories effectively select the right syringe filter to meet their specific sterilization needs while adhering to stringent regulatory standards?
This article delves into the essential insights surrounding filter sterilization syringe applications, exploring their benefits, materials, and the pivotal role they play in maintaining the integrity of scientific research.
JM Science Syringe Filters: High-Quality Solutions for Sterilization
JM Science presents an extensive array of premium medical devices meticulously crafted for sterilization purposes. These devices utilize state-of-the-art materials and technologies, ensuring the from samples—an essential requirement for facilities that demand precise and sterile conditions for experiments and analyses.
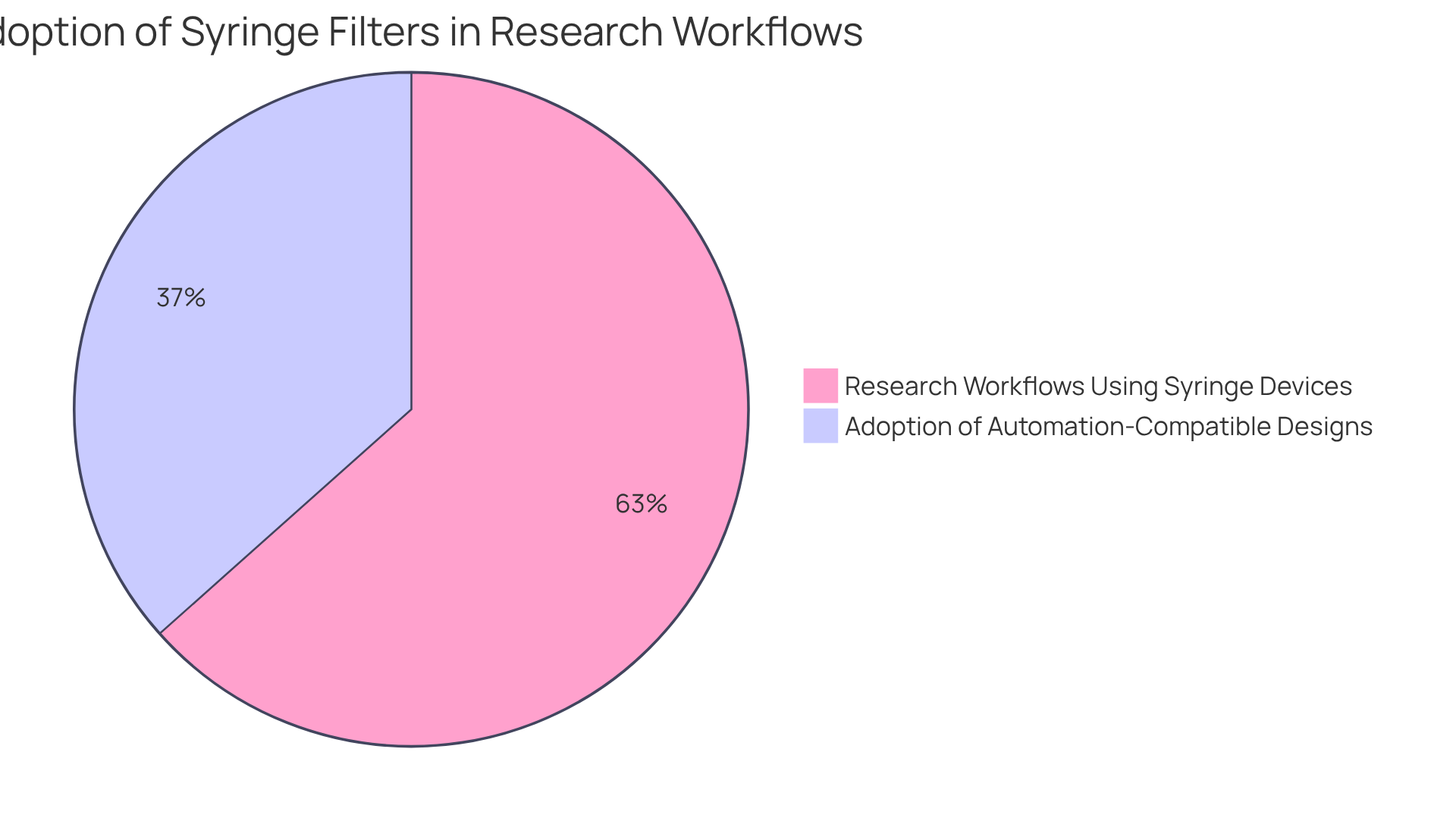
Compatible with a variety of solvents and available in multiple pore sizes, these injection devices cater to specific filtration needs, positioning themselves as indispensable tools in research environments. The escalating emphasis on stringent quality control within the pharmaceutical and biotechnology sectors underscores the necessity for reliable filtration solutions, especially the use of filter sterilization syringes, as over 52% of life science research workflows now incorporate syringe devices.
Moreover, advancements in purification technology, such as the rise of automation-compatible designs—which have seen over 30% adoption in high-throughput facilities—enhance operational efficiency, rendering these devices crucial for modern research practices.
Role of Syringe Filters in Laboratory Sample Preparation and Sterilization
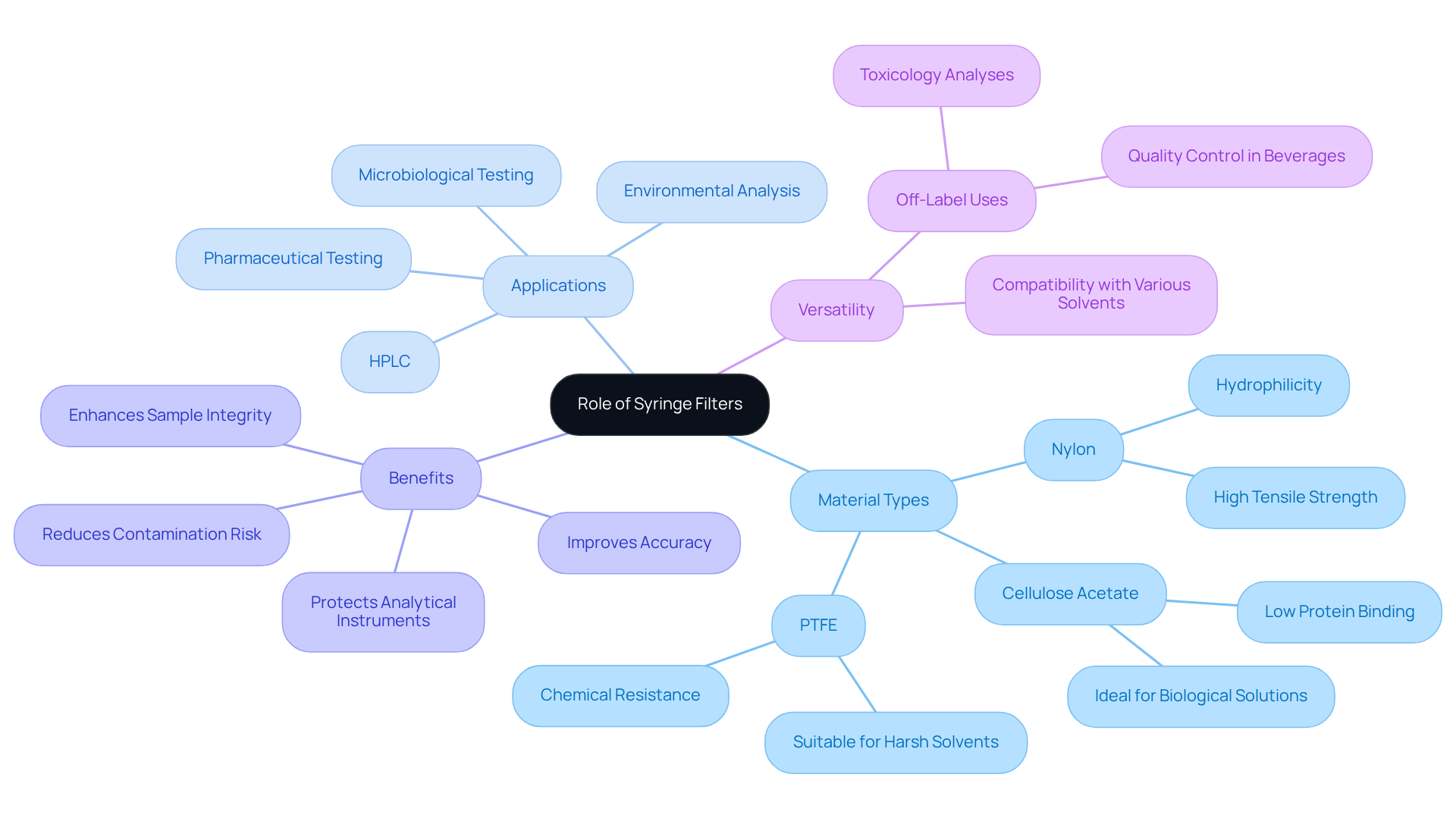
The filter sterilization syringe utilizes syringe membranes that play a pivotal role in laboratory preparation, effectively removing particulates, bacteria, and other impurities from liquid specimens. This filtration process is vital for ensuring that specimens meet the stringent requirements for analysis, particularly when utilizing a filter sterilization syringe in sensitive applications such as [high-performance liquid chromatography (HPLC)](https://hawach.com/news/why-choose-ptfe-syringe-filters-for-hplc-applications.html) and microbiological testing. By utilizing injection devices made from materials like nylon, cellulose acetate, and PTFE, laboratories can leverage their unique properties tailored for specific applications. For instance, nylon membranes excel in purifying liquid specimens, while PTFE membranes are ideal for harsh solvents.
Moreover, the color-coding of injection devices according to membrane type or pore size significantly reduces errors during sample preparation in high-speed environments. By employing a filter sterilization syringe, facilities can substantially enhance their processes, reduce contamination risks, and improve the accuracy of their analytical results. These devices are user-friendly and seamlessly integrate into various scientific protocols, making them indispensable tools in contemporary research. Their function in safeguarding sensitive instruments from clogs and damage further elevates their importance, ensuring reliable and reproducible data across diverse applications.
Additionally, needle meshes may serve off-label purposes, such as filtering impurities from illicit substances for toxicology analyses, showcasing their versatility in different research contexts. To maximize their effectiveness, it is crucial for testing facilities to of syringe membranes with their specific specimens.
Importance of 0.45 Micron Pore Size in Filter Sterilization
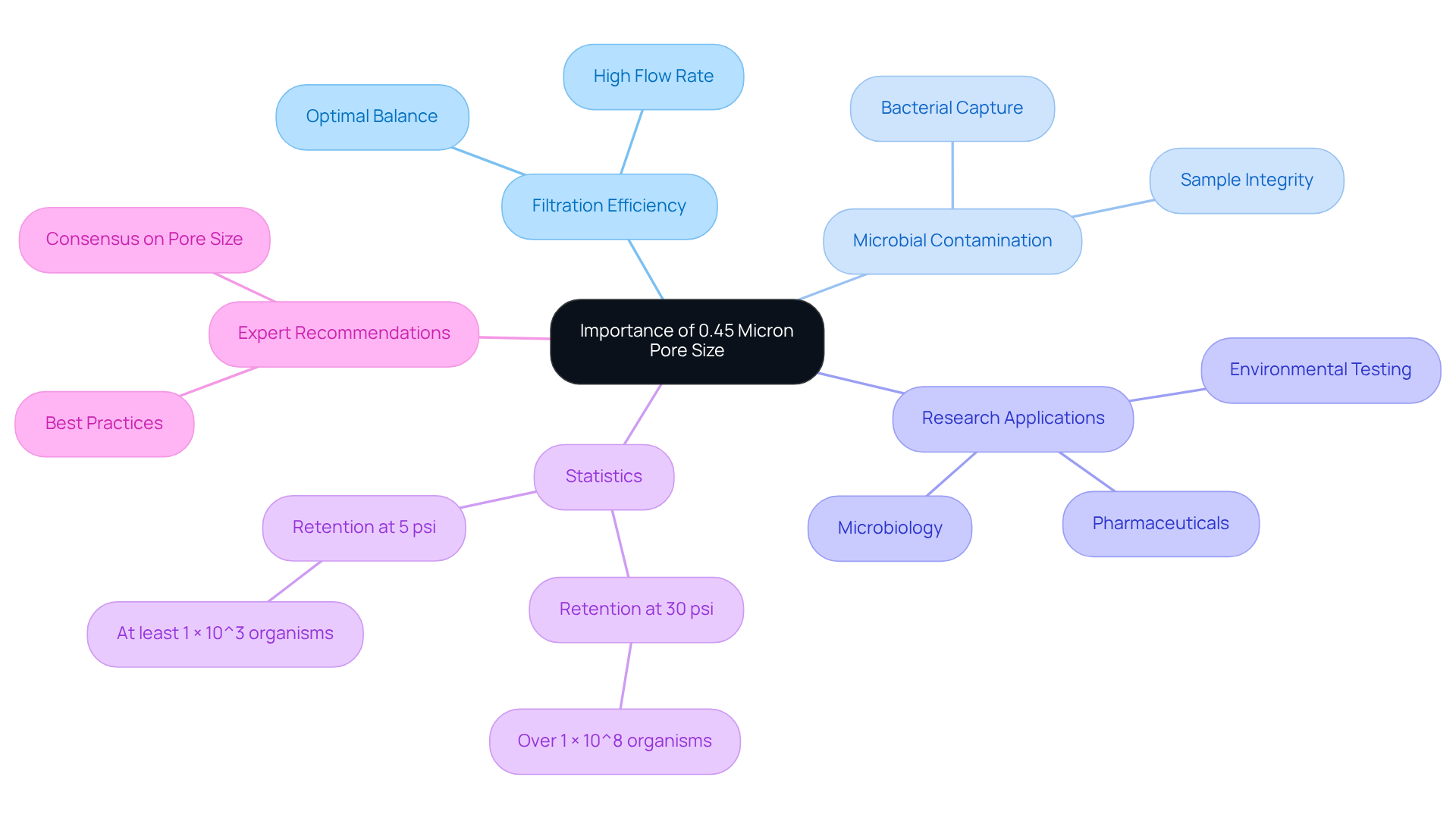
The 0.45 micron pore size is widely acknowledged as the standard for sterilization, particularly in microbiological applications. This size effectively and larger particulates, ensuring that filtered solutions remain devoid of microbial contamination. In research environments, employing a 0.45 micron strainer is essential for preserving sample integrity, especially when preparing solutions for sensitive analyses. This pore size strikes an optimal balance between filtration efficiency and flow rate, making it suitable for a diverse range of experimental applications.
Research indicates that 0.45 micron membranes can retain over 1 × 10^8 organisms at 30 psi and at least 1 × 10^3 organisms at 5 psi, underscoring their efficacy in sterilization processes. Moreover, expert consensus consistently endorses the use of this pore size as a best practice for microbial recovery, with recommendations affirming that the ideal pore size for optimal microbial recovery is 0.45µm. Furthermore, devices must capture over 99% of suspended particles, regardless of particle size or flow rate, thereby reinforcing the reliability of 0.45 micron screens in laboratory settings.
Materials Used in Syringe Filters: Impact on Sterilization Efficacy
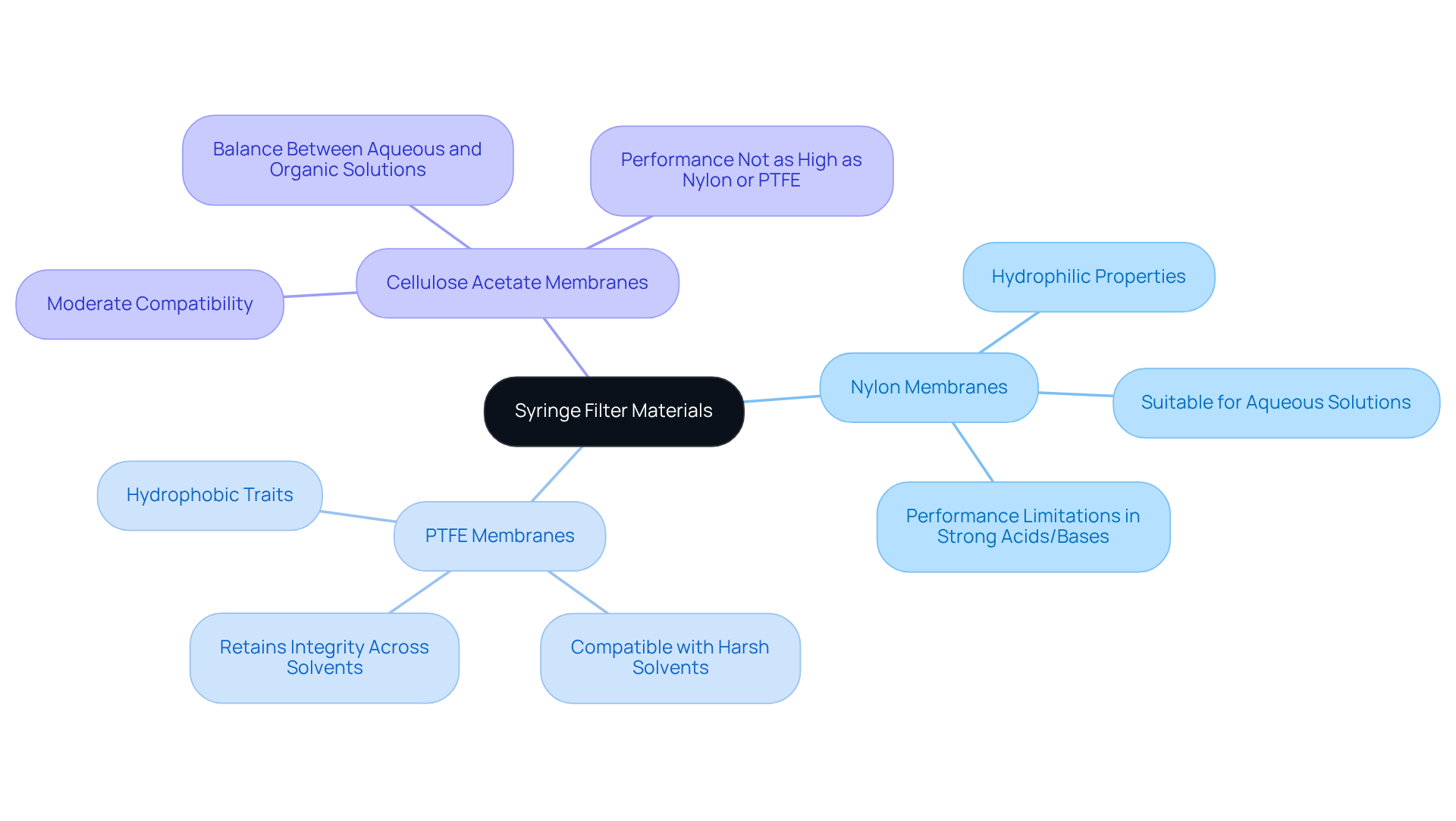
Syringe membranes are composed of various substances, including nylon, PTFE, and cellulose acetate, each possessing distinct characteristics that significantly influence their filtration efficacy.
- Nylon membranes, known for their hydrophilic properties, excel in purifying aqueous solutions, making them a preferred choice for biological samples.
- Conversely, PTFE membranes exhibit hydrophobic traits, rendering them suitable for organic solvents and harsh chemicals commonly encountered in laboratory settings. This compatibility with solvents is critical; while nylon membranes can withstand several solvents, their performance diminishes in the presence of strong acids or bases.
- In contrast, PTFE membranes retain their integrity across a wider range of harsh solvents, ensuring reliable separation without compromising sterility.
- Cellulose acetate membranes strike a balance between the two, providing moderate compatibility with both aqueous and organic solutions, although they may not match the performance of nylon or PTFE under extreme conditions.
- The choice of membrane material directly impacts not only the purification process's effectiveness but also the final product's sterility, which is a vital consideration in pharmaceutical and research environments, particularly when utilizing a filter sterilization syringe.
- Experts emphasize that selecting the appropriate filter sterilization syringe based on specific applications can enhance both the reliability of results and the safety of research practices.
- Understanding these material characteristics is essential for making informed decisions when selecting for various research applications.
Key Advantages of Syringe Filters for Effective Sterilization
Filter sterilization syringes offer significant advantages for efficient sterilization, particularly in laboratory environments where time and precision are paramount. Their innovative design facilitates , allowing for the swift removal of impurities, which is essential for maintaining sample integrity. By enhancing flow rates and filtration capacity, syringe membranes outperform traditional methods.
Furthermore, the disposable nature of these devices minimizes the risk of cross-contamination, ensuring that each specimen remains uncontaminated. Their compact design and compatibility with standard syringes further augment their usability across a range of applications, from routine specimen preparation to specialized research tasks.
Notably, using a filter sterilization syringe with needle membranes featuring a 0.22µm pore size is particularly effective for sterilizing samples in pharmaceutical quality assurance processes. The adoption of single-use practices can drastically lower contamination rates, thereby enhancing the reliability and accuracy of laboratory analyses.
Additionally, needle devices are engineered with advanced pore designs to minimize clogging, which contributes to their overall effectiveness. The syringe membrane market is projected to grow at a CAGR of 6.8% during the forecast period from 2025 to 2034, underscoring their increasing importance within the industry.
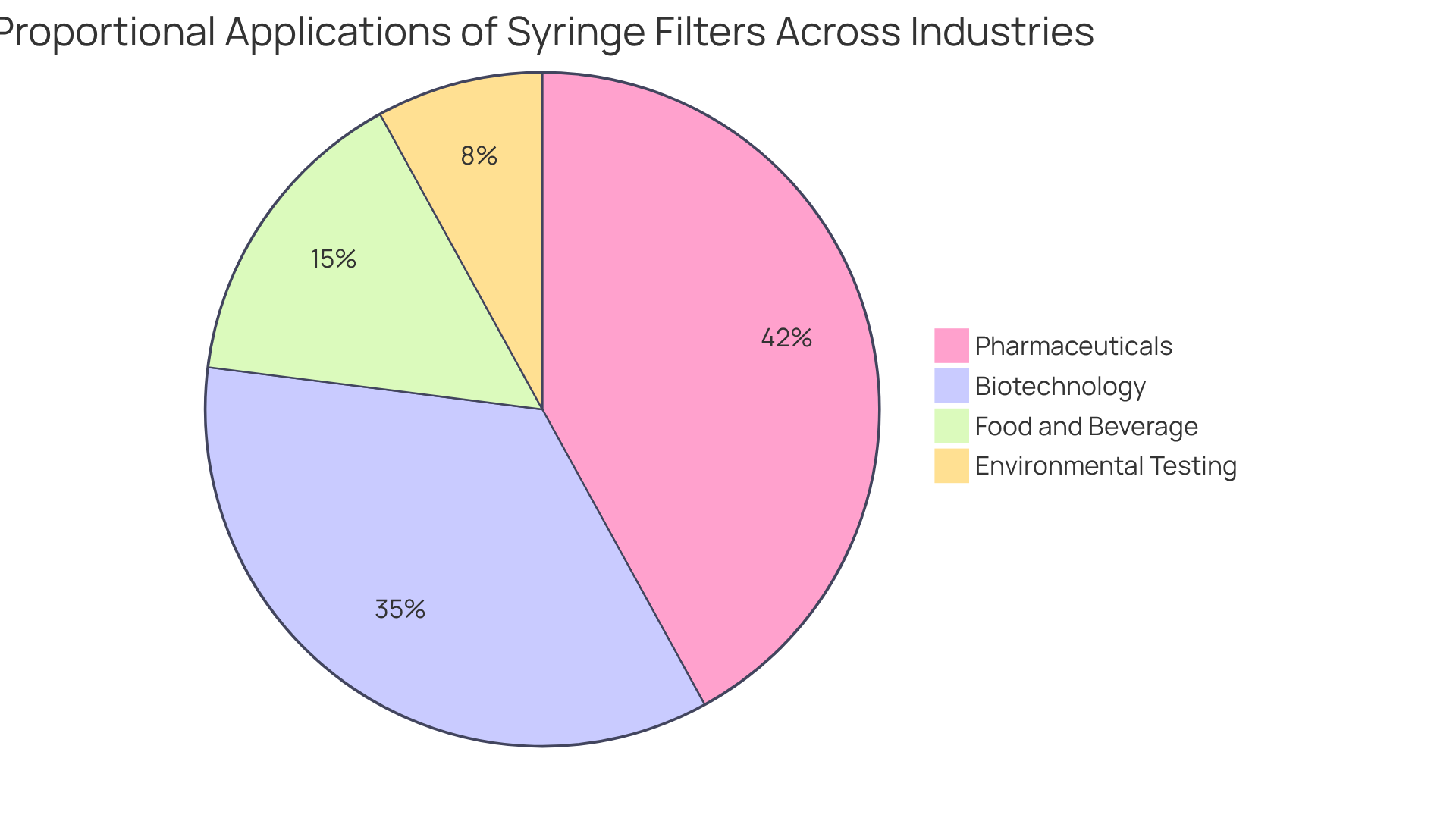
Applications of Syringe Filters in Sterilization Across Industries
Syringe membranes are crucial across multiple industries, including pharmaceuticals, biotechnology, food and beverage, and environmental testing. In the pharmaceutical sector, filter sterilization syringes are essential for sterilizing injectable solutions and ensuring the purity of drug formulations. Notably, over 60% of pharmaceutical-grade applications rely on 0.22 μm membranes to effectively remove contaminants. This specific pore size accounts for more than 42% of the total usage of these devices, underscoring its prevalence in the market. In biotechnology, injection membranes are vital for preparing samples for analysis and maintaining sterile conditions in cell culture processes, with over 65% of sample preparations utilizing these devices to ensure the elimination of particulates and bacteria.
The food and beverage sector also significantly relies on specialized screens to uphold product safety and quality by efficiently removing impurities from liquids. Recent trends reveal that over 48% of funding in the consumables segment is directed toward filtration products, highlighting the increasing focus on quality control in food safety. Moreover, the rising application of injection devices, such as filter sterilization syringes, in life sciences and biotechnology research is driven by innovations in drug development and biopharmaceuticals, emphasizing their versatility as essential tools in maintaining high standards of quality and safety across these fields.
As the global market for injection devices is projected to exceed USD 27.70 billion by 2031, with a CAGR of 6.72% throughout the forecast period, the demand for these products is expected to continue growing. This trend is particularly influenced by and the necessity for accurate, contaminant-free outcomes in research and production processes. Additionally, the increasing demand for point-of-care diagnostics in North America is reshaping market dynamics, further underscoring the importance of syringe membranes in preserving the purity of specimens.
Syringe Filters vs. Other Filtration Options: Choosing the Right Method
Selecting the appropriate filtration method is crucial, as it directly impacts the specific requirements of the application. Syringe separators stand out for small-volume purification due to their user-friendly design and rapid processing capabilities. They prove particularly effective in laboratory settings for tasks such as sample preparation and quality control, where swift results are paramount. For instance, in pharmaceutical manufacturing, a filter sterilization syringe is routinely employed to ensure the sterility of injectable solutions, underscoring its vital role in maintaining product quality. The Single-use Sterile Syringe Filters market has experienced significant growth, propelled by the rising demand for contamination-free solutions, with historical market sizes from 2019 to 2023 reflecting a robust trend.
Conversely, larger-scale applications may derive benefits from membrane separations or centrifugation methods. Membrane filters excel in handling substantial volumes and are frequently utilized in environmental testing to analyze large quantities of water or air samples for contaminants. However, they may not offer the same ease of use as needle devices for quick, point-of-use tasks. For example, while membrane screens are ideal for microbiological testing in the food and beverage industry, where pore sizes of 0.22 µm and 0.45 µm are commonly employed, injection devices provide a more efficient solution for urgent filtration needs.
Understanding the strengths and weaknesses of each is essential for selecting the most appropriate solution for scientific requirements. The decision between a filter sterilization syringe and membrane filters should take into account factors such as volume, efficiency, and the specific characteristics of the liquids being filtered. By aligning the filtration method with application requirements, research facilities can enhance their operational efficiency and ensure high-quality results. As emphasized by industry experts, the selection of the right filtration method is vital for achieving optimal performance in laboratory processes.
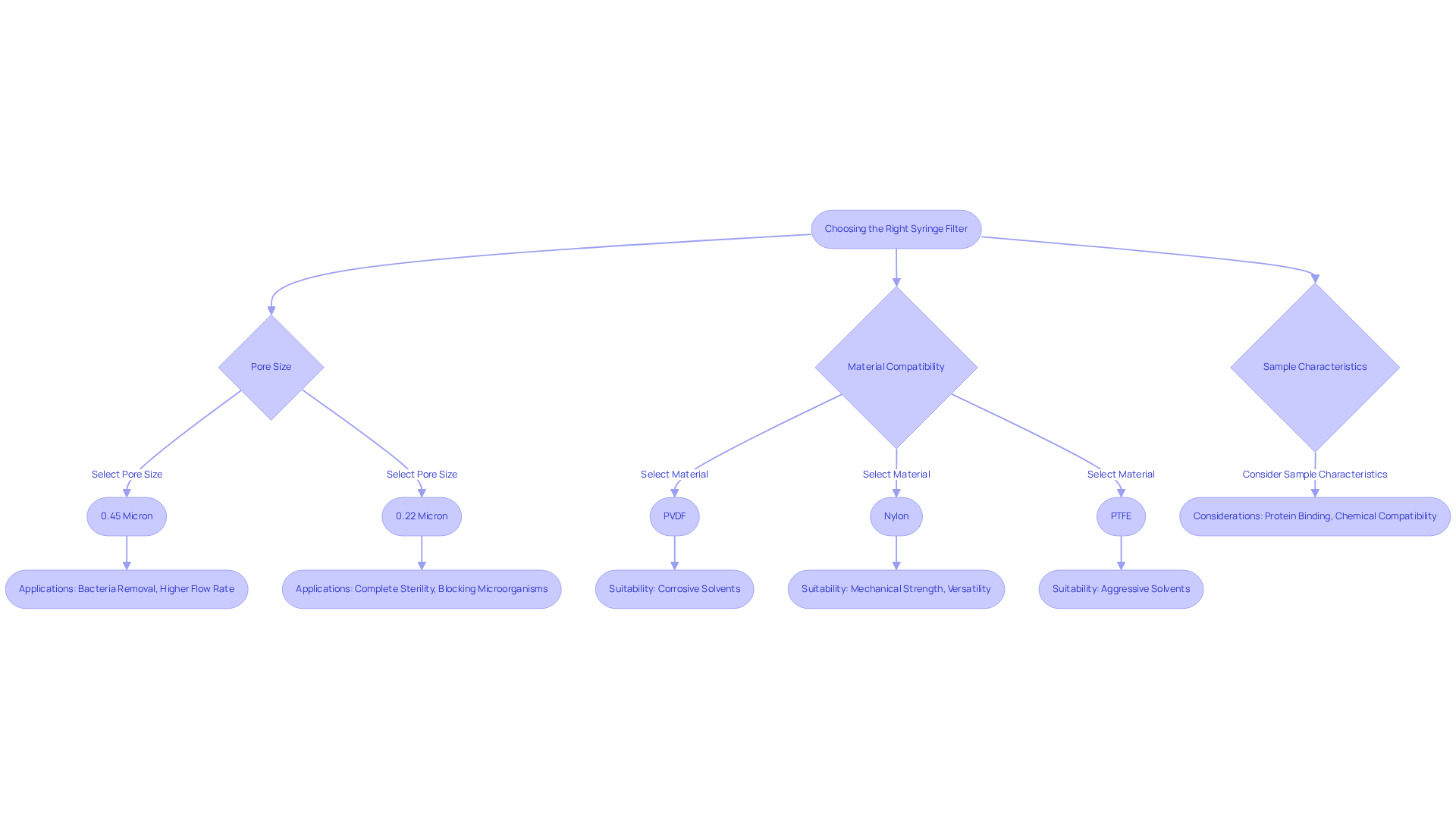
Choosing the Right Syringe Filter for Sterilization Needs
Selecting the appropriate syringe membrane for sterilization requires careful consideration of several critical factors, including pore size, material compatibility, and the specific characteristics of the sample being processed. For general applications, a 0.45 micron screen effectively removes bacteria and larger particulates, providing a higher flow rate than 0.22 micron screens. This advantage is particularly beneficial for rapid sterilization or clarification. However, when complete sterility is essential, a 0.22 micron membrane is necessary, as it is renowned for its superior ability to block bacteria, smaller microorganisms, and viruses, achieving high levels of sterility crucial in pharmaceutical and biotechnology processes. Notably, 0.2 µm membranes are specifically utilized for sterile separation, underscoring their importance in these applications.
Material compatibility is another vital consideration; choosing a device made from materials such as ensures resistance to corrosive solvents, which is crucial for maintaining the integrity of the sample. For example, PVDF syringe membranes are identifiable by a yellow color-coded ring for easy recognition. In contrast, nylon variants, celebrated for their mechanical strength and versatility, are suitable for a broad range of applications but may not be ideal for strong acids. Conversely, PTFE membranes excel in aggressive solvent environments, making them suitable for critical filtration tasks. It is also essential to consider membranes with low protein-binding characteristics, such as cellulose acetate or PVDF, to help preserve specimen integrity during critical experiments.
Real-world applications highlight the significance of these considerations. In pharmaceutical manufacturing, 0.22 micron membranes are routinely employed to uphold product sterility and quality, serving as a primary barrier against contaminants. Furthermore, the use of sterile needle screens is recommended to minimize contamination risks during biological sample preparation. By meticulously evaluating these factors, laboratory professionals can select the most efficient filter sterilization syringe tailored to their sterilization needs, ensuring adherence to industry standards and safeguarding the integrity of their work. Additionally, ISO and CE certifications provide researchers with assurance that the filtration devices comply with international standards for reliability and safety, further enhancing the credibility of their selection.
Regulatory Compliance and Standards for Syringe Filter Sterilization
Laboratories must comply with stringent regulatory standards when utilizing a filter sterilization syringe. Adherence to guidelines established by the FDA and ISO is essential for guaranteeing the safety and effectiveness of filtered products. This compliance involves:
- The proper validation of filtration processes
- Routine maintenance of equipment
- Meticulous documentation of all procedures
By diligently adhering to these regulations, facilities can uphold high-quality standards, ensuring that their sterilization practices are both effective and dependable. Furthermore, the FDA's Process Validation Guideline underscores the necessity of statistical methods in validating these processes, reinforcing the significance of data-driven approaches in maintaining compliance. Real-world examples demonstrate that facilities successfully implementing these guidelines not only enhance their but also contribute to improved patient safety and product integrity.
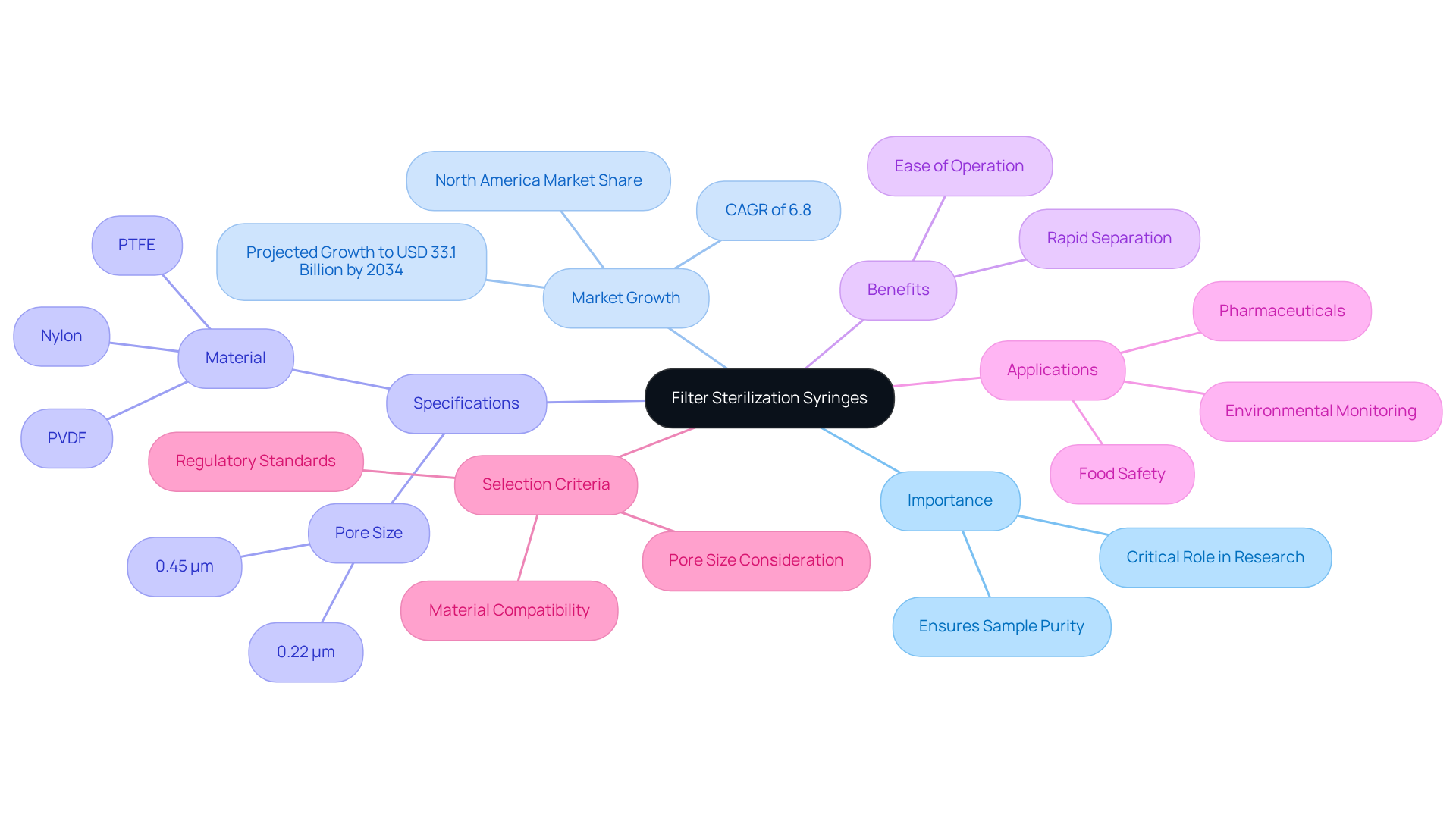
Essential Facts About Filter Sterilization Syringes: A Summary
In conclusion, needle devices such as the filter sterilization syringe are indispensable instruments in research environments, playing a critical role in efficient sterilization and sample preparation. JM Science stands out by providing high-quality filter sterilization syringes designed for diverse applications, ensuring reliability and precision in laboratory processes. Furthermore, the global syringe strainer market is projected to grow from USD 17.6 billion in 2024 to USD 33.1 billion by 2034, with a compound annual growth rate (CAGR) of 6.8%, highlighting the increasing demand for these essential tools.
The standard 0.45 micron pore size is widely recognized for effective sterilization, making it the preferred choice in numerous settings. Material selection is another crucial factor influencing performance and compatibility; Nylon and Polyvinylidene fluoride (PVDF) are the most frequently utilized materials in the market. Additionally, syringe membranes provide significant benefits, including ease of operation and rapid separation, which enhance laboratory processes. Their applications span various sectors, particularly pharmaceuticals and food safety, where maintaining the integrity of specimens is paramount. As emphasized by industry specialists, "Syringe membranes play a crucial role in ensuring sample purity for rapid diagnostic testing."
When selecting the , it is essential to consider factors such as pore size, material compatibility, and adherence to regulatory standards. This careful consideration ensures optimal performance and underscores the importance of high-quality scientific instruments in laboratory settings.
Conclusion
The significance of filter sterilization syringes in laboratory settings is paramount. These devices are essential tools for ensuring the purity and integrity of samples across a wide range of applications, including pharmaceuticals and biotechnology. By utilizing advanced materials and precise pore sizes, filter sterilization syringes effectively eliminate contaminants, thereby safeguarding the accuracy of analytical results and ensuring compliance with stringent regulatory standards.
Key insights explored throughout this article include:
- The crucial role of the 0.45 micron pore size in microbial filtration
- The impact of various materials on sterilization efficacy
- The increasing demand for these devices across diverse industries
The versatility of syringe filters, combined with their user-friendly design and rapid filtration capabilities, underscores their significance in modern research and quality assurance processes. As the market for syringe filters continues to grow, the importance of selecting the right device tailored to specific applications becomes even more critical.
In summary, the careful selection of filter sterilization syringes is essential for achieving optimal results in laboratory practices. By understanding the nuances of pore size, material compatibility, and regulatory compliance, professionals can enhance operational efficiency and ensure high-quality outcomes. Adopting these best practices not only contributes to the advancement of scientific research but also highlights the vital role that syringe filters play in maintaining safety and reliability standards across various industries.




