Overview
This article delivers essential insights into the significance and advantages of PES syringe filters for lab managers. It emphasizes their low protein-binding characteristics, chemical compatibility, and cost-effectiveness. Understanding these benefits is crucial for lab managers aiming to optimize their filtration processes while ensuring compliance with industry standards.
By grasping the nuances of pore size selection, sterilization methods, and best practices for handling and storage, lab managers can enhance operational efficiency and maintain high-quality standards in laboratory settings.
Introduction
The rising demand for high-quality filtration solutions in laboratory settings underscores the crucial role of PES syringe filters in safeguarding sample integrity and adhering to stringent regulatory standards. Lab managers aiming to enhance their purification processes will benefit from recognizing the distinct advantages of PES membranes—characterized by low protein binding and outstanding chemical compatibility—which can markedly improve operational efficiency.
As the laboratory filtration landscape continues to evolve, the challenge remains: how can managers effectively navigate the complexities of selecting the appropriate filters while ensuring compliance and maximizing cost-effectiveness?
JM Science Inc.: Leading Supplier of PES Syringe Filters for Laboratories
JM Science Inc. stands as a prominent provider of PES syringe filters, delivering high-quality products that meet the stringent demands of laboratory environments. These membranes are particularly valued for their low protein-binding characteristics, making them ideal for sensitive applications in biotechnology and pharmaceuticals. By ensuring precision and reliability, JM Science equips lab managers with superior filtration solutions that significantly enhance operational capabilities across diverse scientific applications.
The surge in demand for PES syringe filters within scientific research is propelled by an increasing emphasis on quality assurance and regulatory compliance in laboratory processes. Recent statistics indicate that the global market for syringe strainers was valued at USD 16.4 billion in 2023, with projections suggesting it will reach USD 28.3 billion by 2032, reflecting a compound annual growth rate (CAGR) of 6.2%. PES membranes play an essential role in this growth, effectively preserving sample purity.
Successful applications of PES syringe filter devices in laboratory settings have demonstrated their ability to streamline workflows and enhance analytical outcomes. Laboratories utilizing these devices report greater efficiency in sample preparation, which is critical for high-throughput screening and other advanced research methodologies.
Moreover, ongoing advancements in PES syringe filter purification technology continue to elevate their performance, with innovations aimed at increasing efficiency and expanding their range of applications. Industry leaders emphasize the importance of quality in scientific filtration, asserting that reliable filtering systems are vital for achieving accurate and consistent research results. As industry expert Shruti Bhat articulates, "This robust growth can be attributed to increasing demand in various sectors, including pharmaceuticals and biotechnology, where syringe devices are indispensable tools for sterilizing and clarifying samples." JM Science's unwavering commitment to excellence and innovation positions it as a trusted partner for facilities striving to enhance their purification processes.
Polyethersulfone (PES) Filters: Key Benefits for Laboratory Use
PES syringe filters are highly esteemed in laboratory applications due to their exceptional performance characteristics. They offer significant advantages, including:
- Remarkable thermal stability
- Extensive chemical compatibility
- Minimal protein binding—qualities that are crucial for critical separation processes.
PES membranes are capable of enduring rigorous sterilization techniques, ensuring their integrity and performance are upheld over time. This durability positions PES syringe filters as a preferred choice among laboratory managers who aim to enhance their purification systems.
Recent research has illustrated the efficacy of PES devices across various scientific applications, demonstrating their capacity to efficiently eliminate contaminants while safeguarding sample integrity. For instance, PES membranes have been successfully utilized in pharmaceutical research, where stringent regulatory standards necessitate reliable separation solutions. Compared to alternative materials like nylon and PVDF, PES membranes showcase superior chemical resistance and reduced protein binding, rendering them particularly suitable for biological and aqueous solutions.
Laboratory managers have acknowledged the advantages of PES membranes, with one noting that their low protein binding properties significantly enhance the accuracy of analytical results. Additionally, the thermal stability of PES membranes allows them to function effectively across a wide range of temperatures, further augmenting their adaptability in experimental settings. As the demand for high-quality purification solutions continues to rise, PES syringe filters emerge as a reliable option for optimizing research processes.
Understanding Pore Size: Choosing the Right PES Syringe Filter for Your Needs
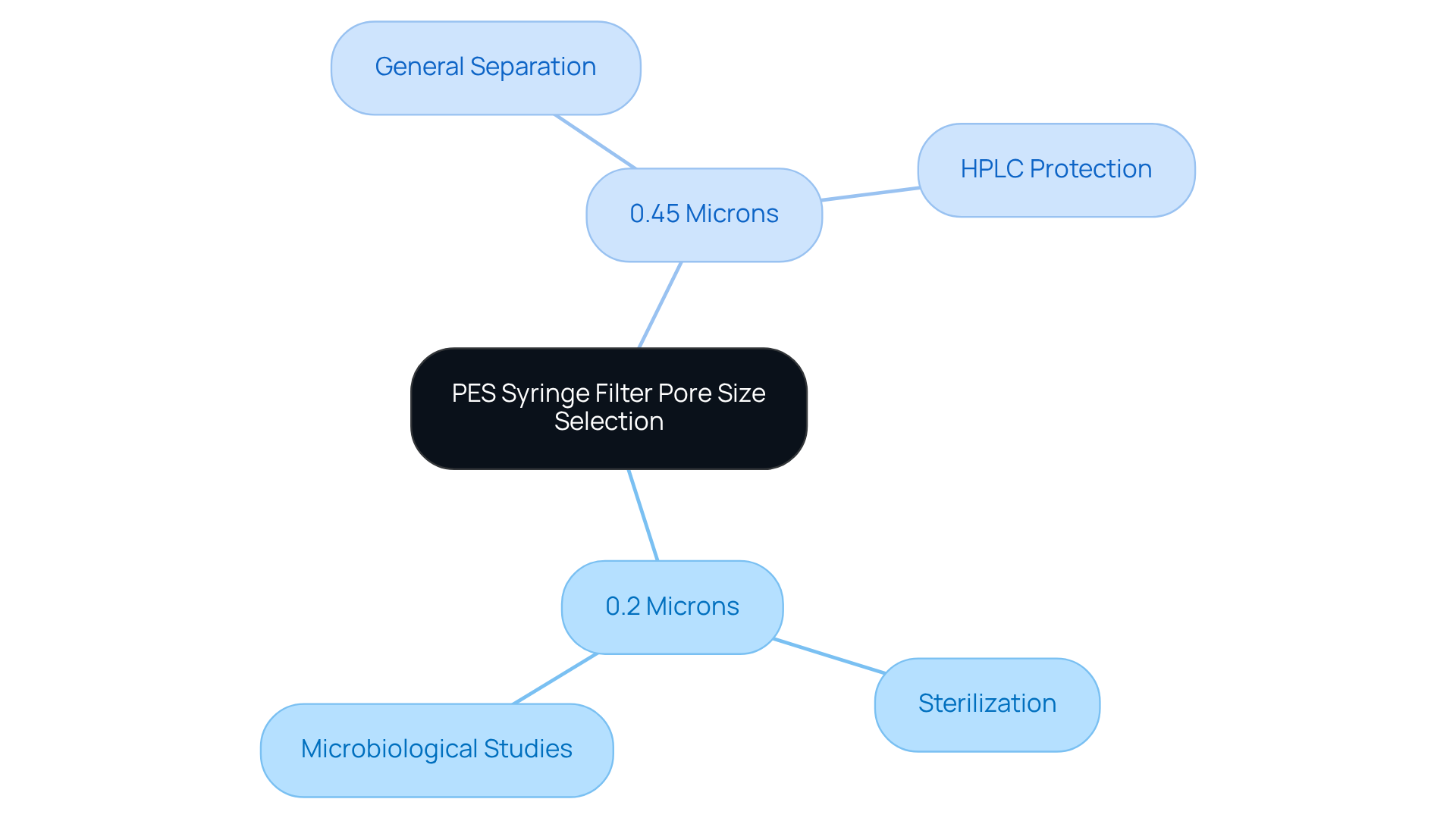
Selecting the appropriate pore size for optimal PES syringe filter performance is crucial for achieving the best filtering results in laboratory environments. Lab managers must assess the specific requirements of their applications, including the nature of the samples being filtered and the desired clarity of the final product. Common pore sizes in a PES syringe filter, typically ranging from 0.2 to 0.45 microns, serve distinct purposes:
- Smaller sizes, such as 0.2 microns, are ideal for sterilizing samples by effectively filtering out bacteria and microorganisms.
- A PES syringe filter with 0.45 microns is versatile for general separation tasks, efficiently removing larger particulates.
The influence of pore size on filtration efficiency is significant. For instance, employing a PES syringe filter with a 0.2 micron membrane is essential in microbiological studies to ensure sample sterility, whereas a 0.45 micron PES syringe filter is often utilized to protect sensitive HPLC instrumentation from particulates that could compromise analytical results. Furthermore, the choice of pore size directly impacts the flow rate and overall processing time; membranes with high flow rates can substantially enhance productivity by facilitating quicker processing of larger sample volumes.
Understanding these specifications is vital for ensuring that the selected apparatus, such as the PES syringe filter, meets the facility's operational needs while maintaining the integrity of the samples. As noted by experts in separation processes, the correct pore size not only improves performance but also contributes to the reliability of analytical results, making it a critical factor in laboratory procedures.
Chemical Compatibility: What Lab Managers Need to Know About PES Filters
Lab managers must prioritize understanding the chemical compatibility of PES syringe filters to prevent complications during filtration processes. PES membranes are typically compatible with a wide range of solvents, acids, and bases. However, it is essential to consult compatibility charts to ensure that the chosen membrane will not deteriorate or release harmful substances into samples. Recent discoveries suggest that improper use of incompatible solvents can cause degradation of the membrane, leading to compromised sample integrity and potential safety risks. For instance, using solvents unsuitable for PES membranes can lead to contamination of the sample.
To guarantee optimal performance, lab managers should routinely assess compatibility information and observe the circumstances in which PES systems are utilized. Maintaining awareness of compatibility is crucial for safeguarding experimental outcomes and adhering to safety regulations, as noted by industry experts. By following these guidelines, facilities can enhance their filtration methods and uphold the quality of their analytical outcomes. Additionally, PES syringe filters are available in various pore sizes, including 0.2 μm, which is recommended for removing smaller particles. This knowledge is essential for maintaining the integrity of experiments and ensuring compliance with safety standards.
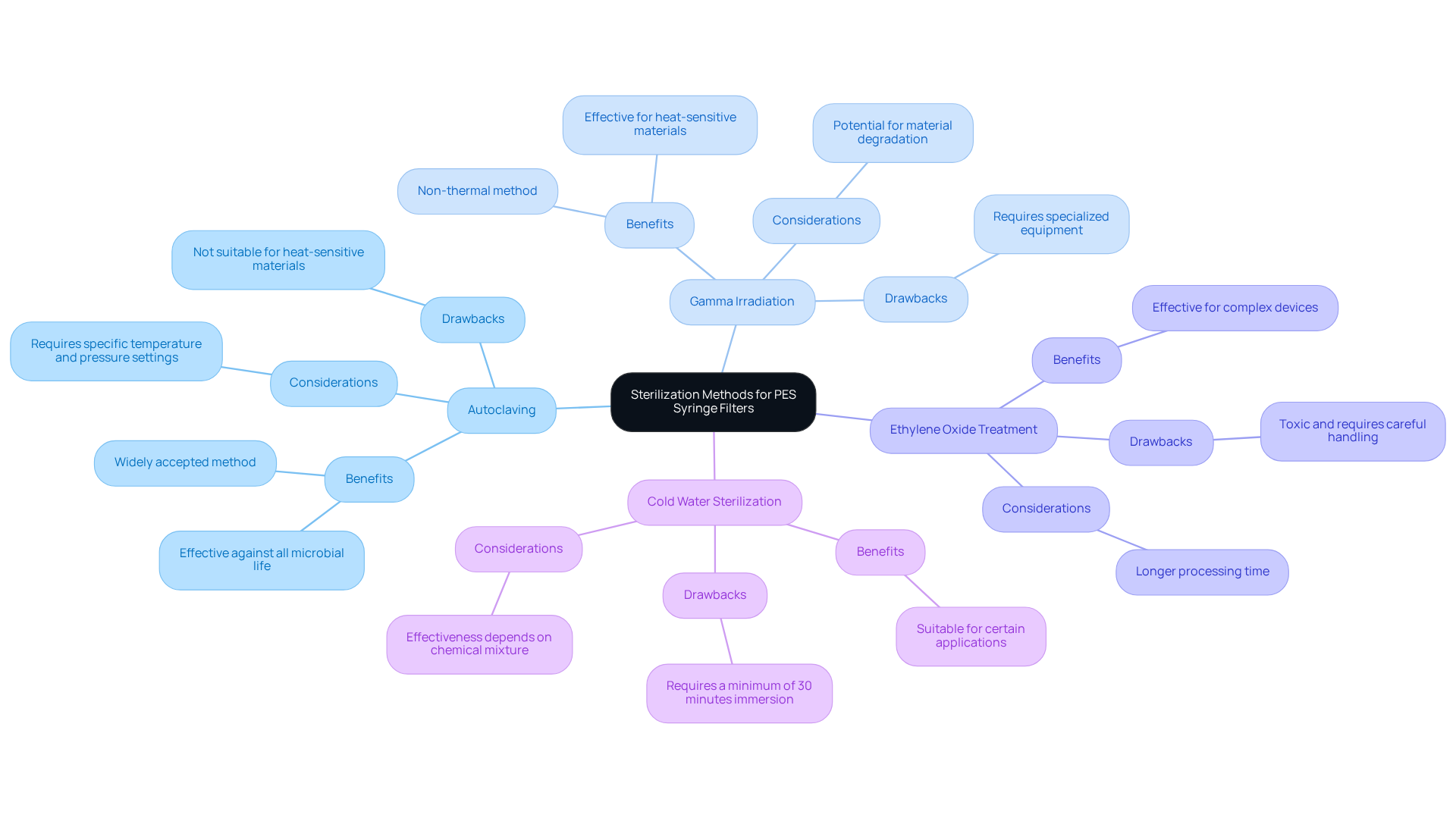
Sterilization Methods for PES Syringe Filters: Ensuring Lab Compliance
Proper sterilization of PES syringe filters is crucial for maintaining laboratory compliance and ensuring safety. The most prevalent sterilization methods include:
- Autoclaving
- Gamma irradiation
- Ethylene oxide treatment
- Cold water sterilization
Each technique offers distinct benefits and drawbacks, necessitating that lab managers select the appropriate method based on the specifications of the device and the properties of the samples being processed. For instance, autoclaving is exceptionally effective in eradicating all forms of microbial life, while gamma irradiation presents a non-thermal option suitable for heat-sensitive materials. Although ethylene oxide treatment is effective, it requires careful handling due to its toxic nature. Cold water sterilization, involving a blend of chemicals and cold water, is effective for certain applications but mandates a minimum of 30 minutes of immersion.
Adhering to proper sterilization procedures not only prevents contamination but also enhances the durability of the PES syringe filter. Compliance issues may arise if sterilization methods are improperly executed, potentially compromising results and creating safety hazards. For example, failure to document sterilization processes or deviations from established protocols can lead to non-compliance during audits. To ensure compliance, lab managers must regularly review sterilization guidelines and maintain comprehensive documentation of sterilization processes. Engaging with laboratory compliance officers can yield valuable insights into best practices for sterilization, underscoring the critical importance of these protocols in laboratory environments.
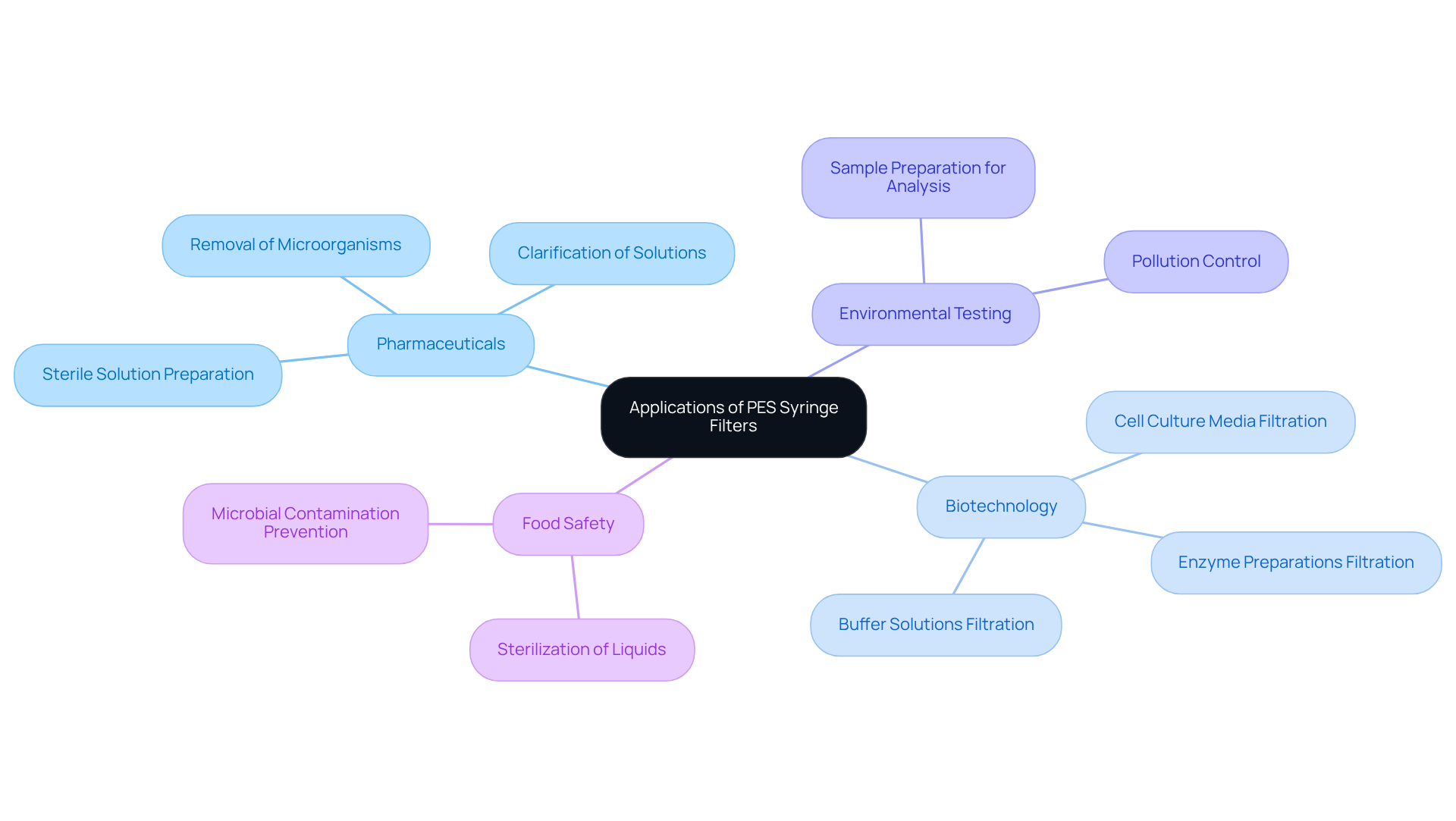
Applications of PES Syringe Filters Across Various Scientific Fields
PES syringe filters are integral components across various scientific disciplines, including pharmaceuticals, biotechnology, environmental testing, and food safety. Their ability to efficiently separate particulates and microorganisms is vital for preparing samples for analysis, thereby ensuring that results are both precise and trustworthy.
In pharmaceutical laboratories, for instance, PES syringe filters are routinely utilized in the preparation of sterile solutions, highlighting their essential role in maintaining product quality. This highlights the critical importance of high-quality scientific instruments in laboratory settings, reinforcing the need for reliable tools to achieve accurate outcomes.
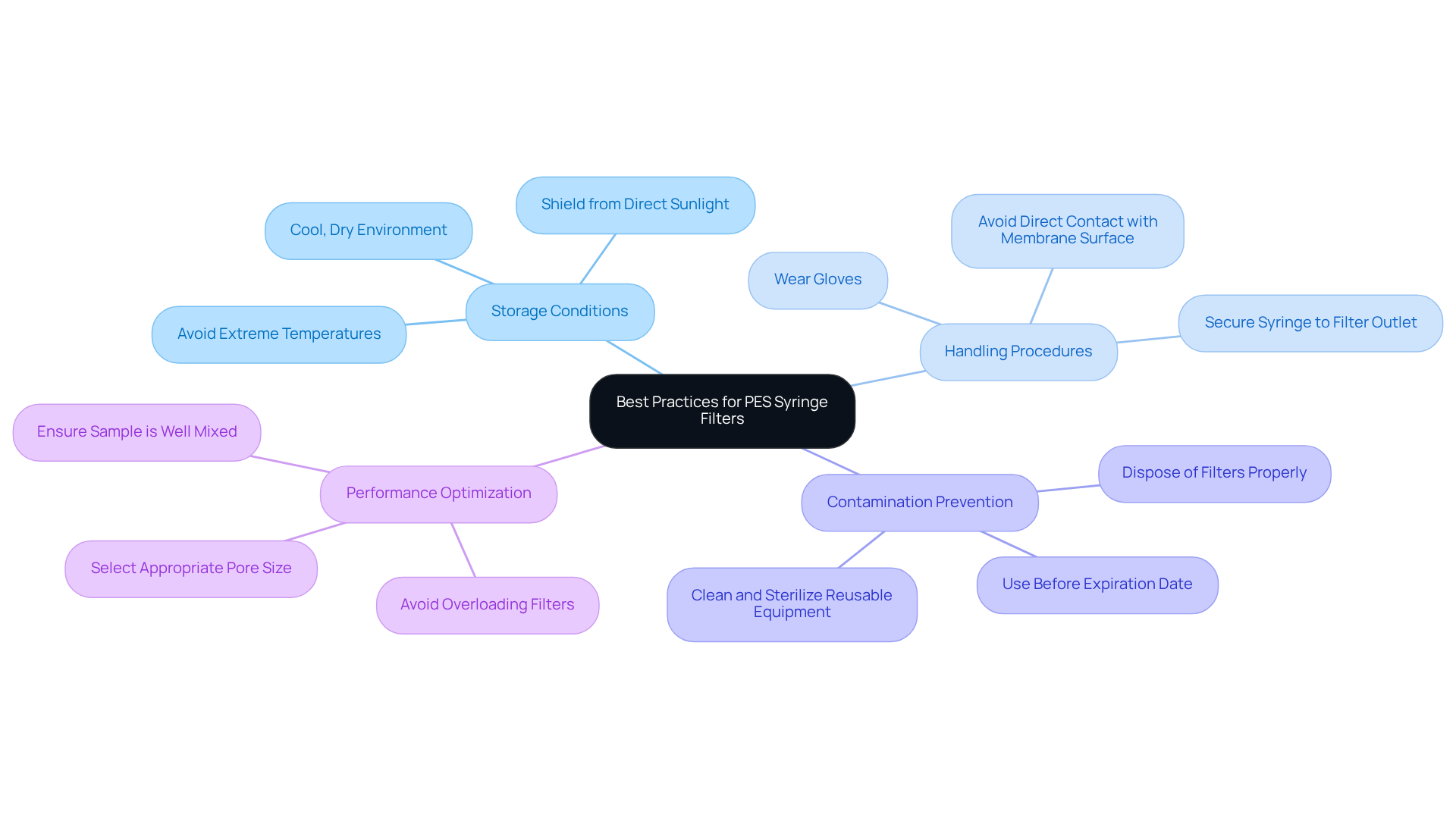
Best Practices for Handling and Storing PES Syringe Filters
To enhance the durability and efficiency of PES syringe filters, laboratory managers must adopt optimal methods for handling and storage. These membranes should be stored in a cool, dry environment, shielded from direct sunlight and extreme temperatures, which can compromise their integrity. Proper handling is paramount; gloves should be worn to prevent contamination, and care must be taken to avoid direct contact with the membrane surface. Additionally, filters should be utilized before their expiration date to ensure optimal performance.
Sterile syringe membranes play a critical role in pharmaceutical research, purifying drug samples prior to analysis and ensuring both purity and accuracy. Improper storage can result in contamination issues, such as microbial growth or chemical degradation, significantly impacting the accuracy of test results. Both 0.2 and 0.22 micron membranes are suitable for critical applications requiring sterilization. Therefore, maintaining appropriate storage conditions is essential for preserving the functionality of PES membranes and ensuring reliable results in laboratory analyses when using a PES syringe filter.
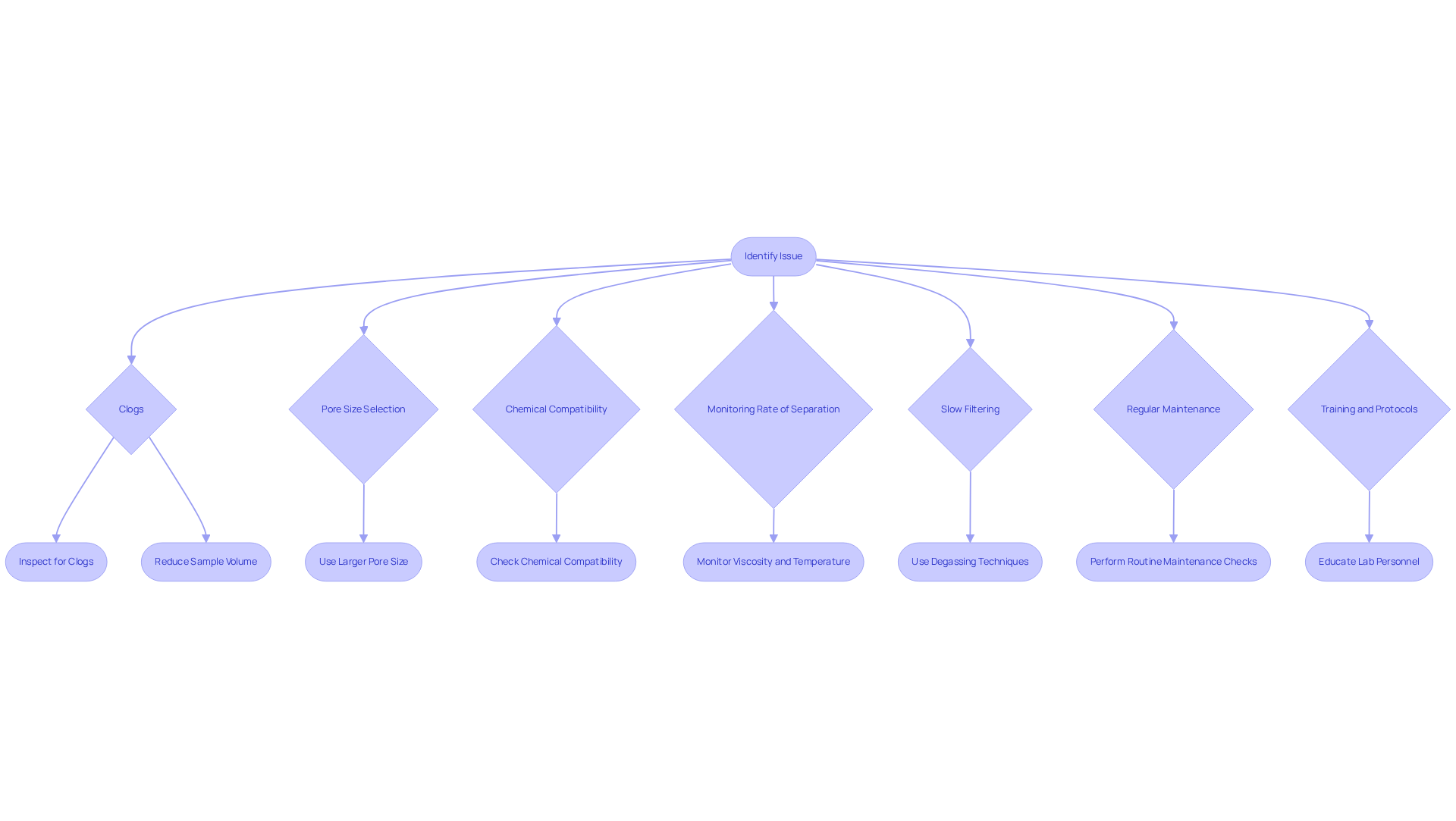
Troubleshooting PES Syringe Filters: Common Issues and Solutions
Lab managers often face challenges with the PES syringe filter components, particularly concerning slow filtration rates and potential contamination. To effectively tackle these issues, it is crucial to implement several strategic measures.
-
Check for Clogs: Regular inspection of filters for blockages is essential, as clogs can significantly impede flow. This common issue can be mitigated by reducing sample volume or using screens with larger pore sizes when dealing with larger particles.
-
Pore Size Selection: It is vital to ensure that the chosen pore size aligns with the sample being filtered. Employing a membrane with a pore size that is too small can lead to diminished flow rates and inefficient filtration.
-
Chemical Compatibility: Confirming that the materials used are compatible with the solvents and samples is paramount. Chemical incompatibility can lead to unexpected contamination and failures in the filtration process, undermining laboratory integrity.
-
Monitoring Rate of Separation: The average rates for PES membranes can vary based on factors such as sample viscosity and temperature. Keeping a close watch on these parameters enables timely adjustments when necessary.
-
Troubleshooting Slow Filtering: Should slow filtering persist, consider utilizing degassing techniques to eliminate trapped air bubbles that may disrupt flow. Additionally, gently tapping the syringe or membrane can help dislodge any air pockets, enhancing performance.
-
Regular Maintenance: Conducting routine maintenance checks on filtration systems is essential for optimal performance. This includes integrity assessments like the bubble point test and pressure hold test to confirm that devices are functioning correctly. Regular calibration of equipment used with syringe screens is also advisable to maintain consistent performance.
-
Training and Protocols: Educating lab personnel on proper handling techniques and troubleshooting protocols is crucial for improving operational efficiency and minimizing disruptions.
By proactively addressing these common challenges, lab supervisors can maintain high operational efficiency and ensure reliable outcomes in their purification processes with a PES syringe filter. Notably, North America accounts for approximately 35% of the global syringe market share, driven by the pharmaceutical and biotechnology sectors, underscoring the importance of effective separation practices.
Cost-Effectiveness of PES Syringe Filters: A Financial Perspective for Labs
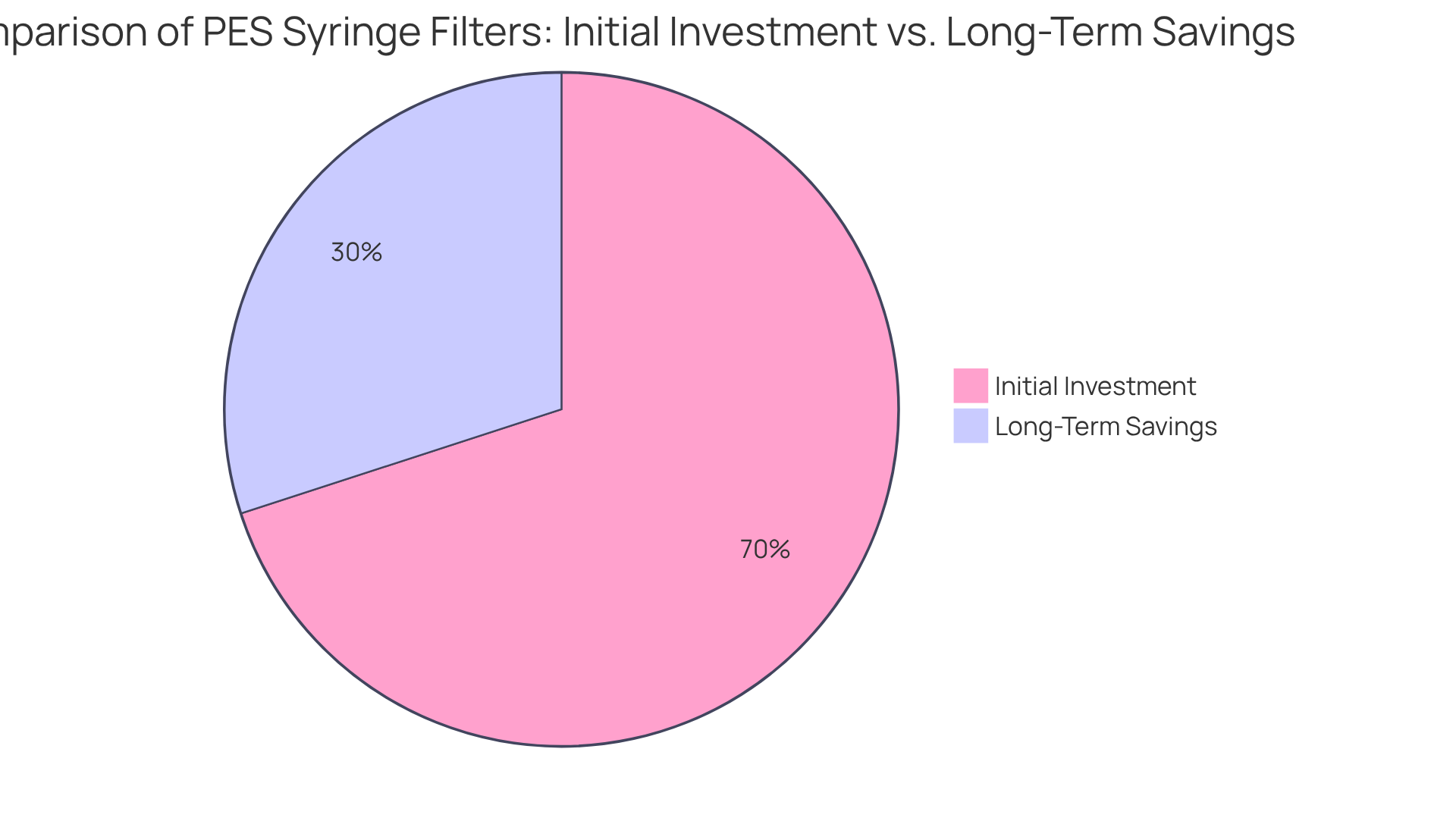
PES syringe filters represent a cost-effective solution for research facilities, primarily because of their extended lifespan and reduced need for replacements. While the initial investment may surpass that of certain other filtration options, the long-term savings can substantially outweigh this upfront cost.
Financial analysts emphasize the importance of assessing the total cost of ownership when selecting laboratory supplies, as PES syringe filters not only mitigate sample loss from contamination but also contribute to lower operational expenses over time.
Research indicates that facilities utilizing PES syringe filters can achieve long-term savings of up to 30% compared to traditional systems, making them a prudent choice for lab managers focused on optimizing budgetary allocations. By investing in PES systems, laboratories can enhance operational efficiency while ensuring high-quality outcomes.
Key Takeaways: Essential Insights on PES Syringe Filters for Lab Managers
In summary, PES syringe filters are an invaluable asset for lab managers, providing a multitude of benefits such as high chemical compatibility, effective filtration, and significant cost savings. Grasping the intricacies of pore size, sterilization methods, and optimal handling practices can markedly enhance laboratory operations. By leveraging these insights, lab managers are empowered to make informed decisions that not only improve efficiency but also ensure compliance with industry standards.
Conclusion
PES syringe filters are indispensable tools for laboratory managers, delivering a combination of efficiency, reliability, and cost-effectiveness that significantly enhances laboratory operations. Their distinctive characteristics, including low protein binding and high chemical compatibility, render them essential for various scientific applications, particularly within the realms of pharmaceuticals and biotechnology.
This article has presented crucial insights, emphasizing the necessity of:
- Selecting the appropriate pore size for specific applications
- Understanding the required sterilization methods for compliance
- Recognizing the importance of proper handling and storage practices
Furthermore, the cost-effectiveness of PES syringe filters has been underscored, illustrating how their long-term savings can substantially benefit laboratory budgets.
Ultimately, the adoption of PES syringe filters can yield improved experimental outcomes and operational efficiencies. Laboratory managers are urged to integrate these insights into their practices, ensuring the utilization of these advanced filtration solutions to uphold high standards of quality and compliance within their laboratories. By doing so, they not only enhance their research capabilities but also contribute to the broader advancement of scientific inquiry.




