Overview
This article presents seven essential insights into the definition and significance of pH meters for laboratory managers. It underscores their critical role in ensuring precise pH measurements, which are vital for a range of scientific applications. By detailing the functionality of pH meters, the necessity for regular calibration and maintenance, and recent technological advancements, the article highlights the diverse applications across various industries. Such insights reinforce the importance of grasping the definition of pH meters for effective laboratory management, ultimately prompting lab managers to prioritize these instruments in their operations.
Introduction
The precision of pH measurement is pivotal in laboratory environments, influencing everything from chemical reactions to product quality. As lab managers navigate the complexities of maintaining accuracy in their analyses, understanding the nuances of pH meters becomes essential. They face significant challenges in selecting the right instruments, ensuring proper calibration, and maintaining optimal performance. This article delves into seven key insights that illuminate the definition of pH meters and their critical role in enhancing laboratory efficiency and reliability.
JM Science pH Meters: Precision Instruments for Laboratory Needs
JM Science presents an extensive range of high-quality pH devices specifically engineered to meet the rigorous demands of research environments. These precision instruments are essential for obtaining accurate pH measurements, which are critical for ensuring reliable results across various applications, including pharmaceuticals, food science, and environmental testing, as described by the pH meter definition.
Featuring advanced capabilities such as temperature compensation and user-friendly interfaces, JM Science pH devices significantly enhance laboratory efficiency and accuracy. Their successful deployment in numerous laboratories underscores their reliability, establishing them as the preferred choice for lab managers who prioritize dependable solutions.
Additionally, the growing demand for portable pH devices reflects a trend towards convenience and simplicity, which is connected to the pH meter definition and the latest advancements in pH technology. Market reports indicate that the pH instruments market is projected to experience impressive growth, driven by high demand across industries such as food & beverage and pharmaceuticals.
As the market for pH meters continues to expand, JM Science remains at the forefront by ensuring its products evolve to meet the changing needs of the scientific community, which aligns with the pH meter definition, while upholding a strong commitment to customer support.
pH: The Measure of Acidity and Alkalinity in Solutions
The pH meter definition highlights that the pH scale is a critical tool for quantifying the acidity or alkalinity of a solution, which ranges from 0 (highly acidic) to 14 (highly alkaline), with 7 indicating neutrality. For lab managers, understanding the significance of pH is paramount, as it directly influences chemical reactions, biological processes, and the stability of various compounds. The pH meter definition emphasizes that precise pH assessment is essential for maintaining the quality and consistency of results across diverse applications.
Recent studies reveal a growing demand for pH sensors in the pharmaceutical industry, underscoring their vital role in ensuring product quality, stability, and effectiveness. As Ron Erickson, Chairman/CEO, emphasizes, "The application of accurate calibration technologies is essential for ensuring the reliability of laboratory results."
To achieve precise pH readings, lab supervisors must conduct regular calibration of their pH devices, which is essential as the pH meter definition shows that even minor fluctuations in pH can significantly impact reaction rates and product stability.
Glass Electrode: The Heart of pH Meter Functionality
The glass electrode serves as a pivotal component of pH meters, tasked with sensing the hydrogen ion concentration in a solution. Its distinctive characteristics facilitate precise assessments, rendering it indispensable in laboratory environments. Recent studies indicate that the slope of glass electrodes can vary from 55.98 to 57.70 mV, closely aligning with the Nernst equation, which underscores their reliability in delivering accurate pH readings. Additionally, the hardness of tap water used in experiments ranged from 332 to 356 mg/L CaCO3, while its conductivity fluctuated between 682 to 904 µS/cm—factors that can significantly influence pH readings.
Laboratories are increasingly enhancing their pH testing processes by adopting advanced glass electrode technology. Innovations, such as improved membrane designs and enhanced resistance to drift, have been developed, enabling more stable and accurate measurements over time. Notably, the potential range between electrodes can expand from approximately 25 mV to 100 mV as they age, highlighting the necessity for regular adjustment and maintenance. Experts recommend calibration at least once a year, typically employing a two-point calibration process with buffer solutions of pH 7.0 and either 4.0 or 10.0.
Despite their advantages, glass electrodes face challenges. Failure rates can be affected by factors such as contamination and improper storage, necessitating diligent care. Consistent cleaning and appropriate storage methods are essential for preserving their functionality and ensuring precise readings. The potential drift of electrodes can range from 11 to 184 µV/day, further emphasizing the need for consistent maintenance.
Experts in the field underscore the significance of glass electrodes as part of the pH meter definition for obtaining reliable pH readings. As one scientist remarked, 'The accuracy of pH devices, as outlined in the pH meter definition, depends on the quality of the glass electrode, making it essential for managers in research settings to prioritize their upkeep and comprehension.' By remaining informed about the latest advancements in glass electrode technology and applying best practices, lab managers can enhance the precision and dependability of their pH readings.
Calibration Procedures: Ensuring Accurate pH Measurements
Routine calibration of pH devices is essential for achieving accurate readings in laboratory environments, as it directly relates to the pH meter definition. Lab managers must adhere to standardized procedures, typically involving the use of buffer solutions with known pH values. Calibration should be performed frequently, especially when the meter is first deployed or after extended periods of inactivity. This practice not only upholds the integrity of evaluations but also ensures compliance with regulatory standards, as evidenced by JM Science Inc.'s impressive 780+ accreditations across 28 metrological and testing areas.
The precision of pH meter definition readings can significantly impact experimental results, as even minor fluctuations in pH can dramatically alter a substance's chemical characteristics. As one expert noted, "Since even minor changes in pH can significantly modify a substance’s chemical characteristics, precise pH assessment is crucial." Regular adjustments are essential for aligning the pH meter's current features with the pH sensor, which helps in understanding the pH meter definition and minimizing issues such as reading drift and aging electrodes. Research indicates that the pH meter definition emphasizes consistent calibration with standardized buffers as vital for ensuring reliable results across various samples.
Moreover, implementing multi-point adjustments allows for tuning at over two pH levels, thereby expanding the device's range and enhancing precision. Laboratories that enforce rigorous measurement protocols frequently report increased accuracy in their pH readings, which is critical for applications ranging from soil analysis to food safety. By prioritizing consistent calibration, laboratory supervisors can ensure their pH devices yield dependable outcomes that align with the pH meter definition, ultimately reinforcing the credibility of their scientific endeavors. JM Science also offers instructional videos and application libraries to support lab managers in effectively executing these calibration protocols.
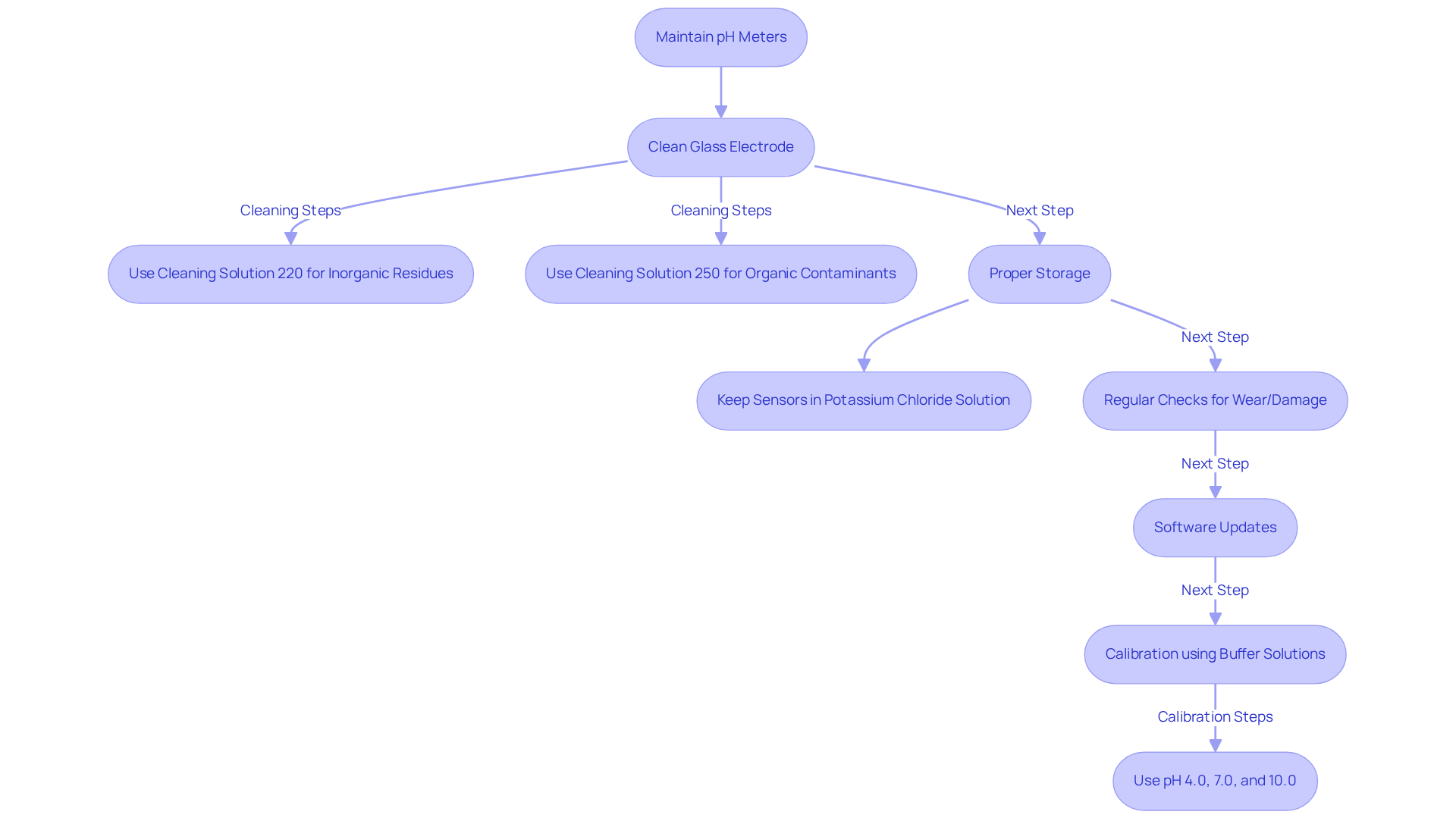
Maintenance Tips: Keeping pH Meters in Optimal Condition
To maintain pH instruments in optimal condition, lab managers must understand the pH meter definition and establish a routine of regular upkeep. This involves:
- Cleaning the glass electrode with appropriate solutions, such as Cleaning Solution 220 for inorganic residues and Cleaning Solution 250 for organic contaminants.
- Proper storage, which is equally crucial; to adhere to the pH meter definition, pH sensors should be kept moist in a potassium chloride solution to prevent damage and ensure accurate readings.
- Regular checks for signs of wear or damage, along with software updates, can further enhance functionality and accuracy.
Research indicates that with diligent care, the average lifespan of pH devices can be significantly extended, often exceeding five years. Laboratories that adopt thorough cleaning and adjustment practices report fewer inaccuracies and longer-lasting equipment. Furthermore, case studies indicate that facilities emphasizing the pH meter definition and equipment upkeep see a significant enhancement in the reliability of readings.
Quotes from industry authorities underscore the importance of this upkeep: "Regular adjustment and cleaning are essential for extending the lifespan of pH sensors and ensuring accurate measurements." It is also vital to calibrate the pH instrument using a two-point calibration method with buffer solutions of pH 4.0, 7.0, and 10.0 to ensure precise readings. By prioritizing these maintenance practices, laboratories can extend the lifespan of their instruments while maintaining consistent and reliable performance in their analyses.
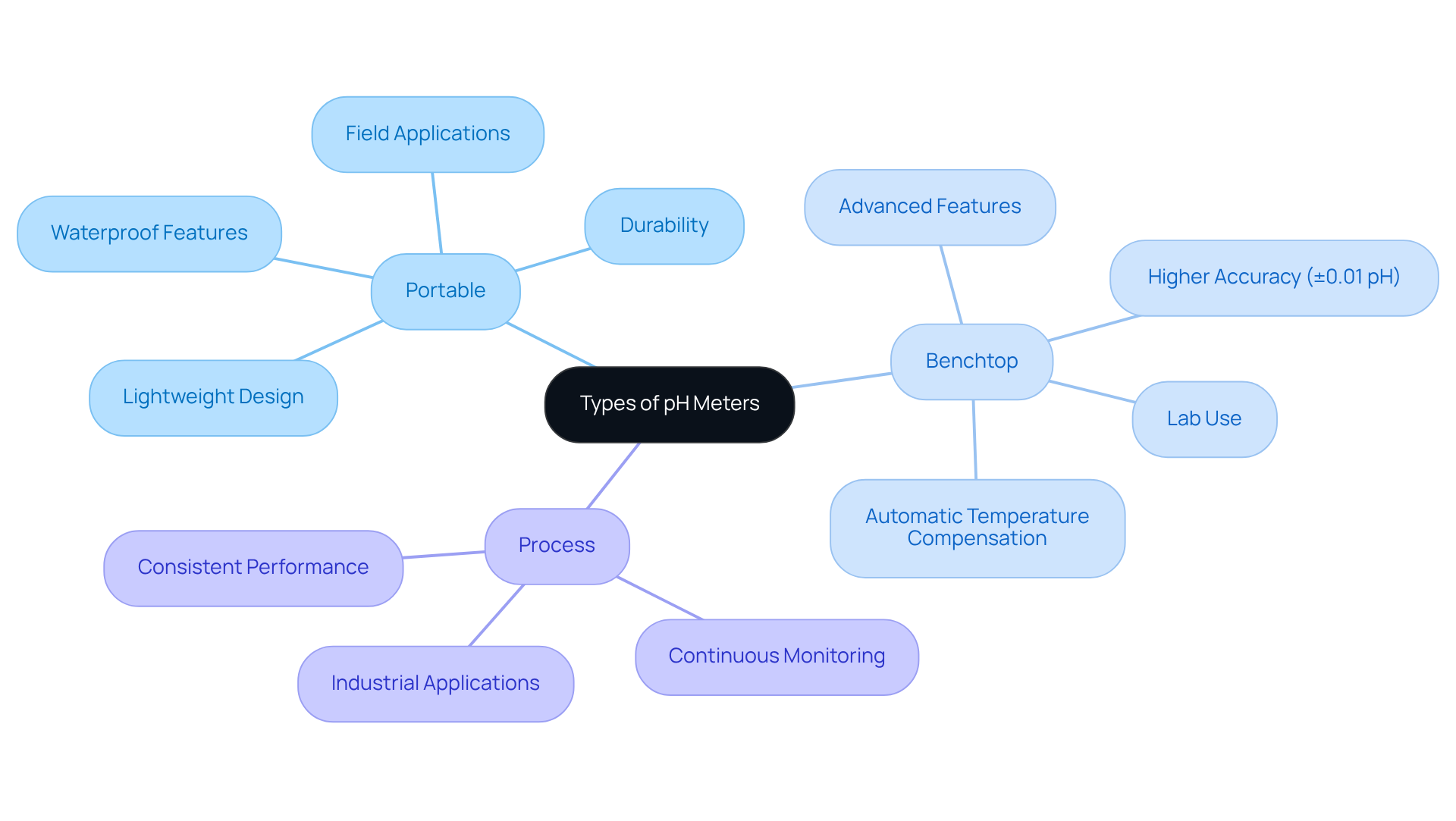
Types of pH Meters: Choosing the Right Instrument for Your Lab
A variety of pH instruments, each tailored for distinct applications such as portable, benchtop, and process devices, can be understood through the pH meter definition. Portable devices excel in fieldwork due to their lightweight design and durability, making them ideal for on-the-go measurements. In contrast, benchtop models are equipped with advanced features, offering higher accuracy and stability—essential for laboratory environments. These devices typically provide an accuracy range of ±0.01 pH, making them perfect for quality assurance and research purposes. Process pH instruments are specifically designed for continuous monitoring in industrial settings, ensuring consistent performance over time.
When selecting a pH device, lab managers must consider several critical factors, including:
- The pH meter definition
- Intended use
- Required accuracy
- Environmental conditions
For instance, if the device will be employed in a laboratory environment, a benchtop model may be preferable due to its enhanced functionality and reliability. Conversely, for field applications, a portable meter that is waterproof and rugged would be more suitable. Additionally, understanding the specific requirements of the laboratory—such as the necessity for automatic temperature compensation, the compatibility of electrodes with the samples being tested, and the cost of replacement electrodes, which are fragile and may require frequent replacement—can significantly influence the decision-making process.
According to the pH meter definition, calibration is crucial for accurate pH measurements and should be conducted using pH buffer solutions, typically at pH 4, 7, and 10. A 2-point calibration is recommended for optimal accuracy, and it is essential to calibrate at the same temperature as the samples being tested, as temperature can impact pH readings. Usage statistics indicate an increasing preference for benchtop pH devices in research environments due to their superior precision and advanced functionalities. However, portable devices continue to be favored for their convenience across various applications. Ultimately, the selection of a pH device should align with the facility's specific requirements, ensuring optimal performance and reliability in readings.
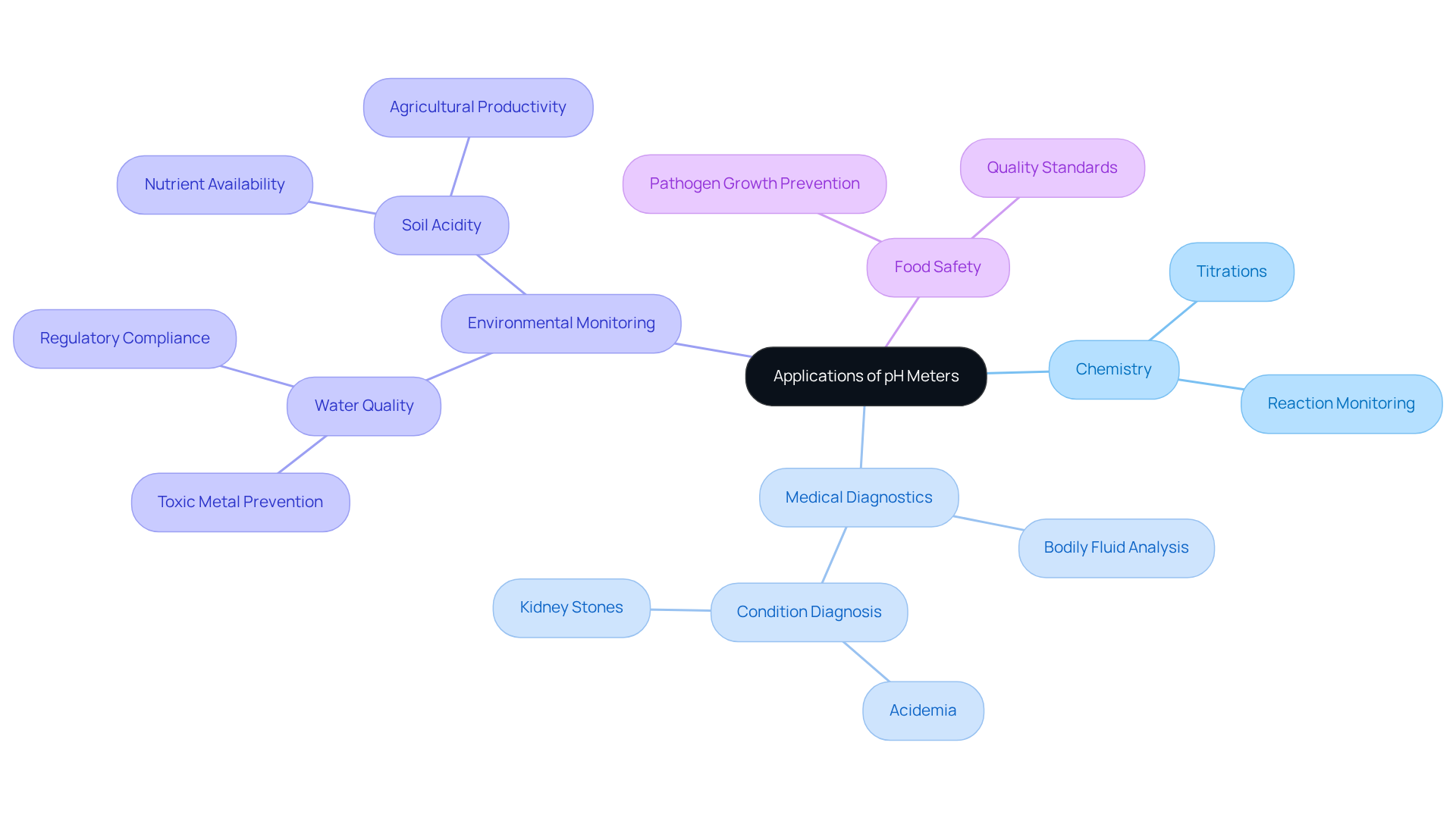
Applications of pH Meters: From Chemistry to Medical Diagnostics
The pH meter definition highlights the importance of pH devices across a range of scientific disciplines, including chemistry, biology, medical diagnostics, and particularly, environmental monitoring. In chemistry laboratories, these instruments are indispensable for titrations and monitoring reactions, providing precise measurements essential for experimental accuracy. Within medical settings, pH devices are employed to analyze bodily fluids, aiding in the diagnosis of conditions such as acidemia and kidney stones.
In the realm of environmental monitoring, pH devices are critical for assessing water quality, a factor crucial for both ecological health and compliance with regulatory standards. For example, maintaining appropriate pH levels in drinking water is essential to prevent toxic metal exposure, with ideal ranges typically falling between 6.5 and 8.5. Additionally, pH instruments are utilized to evaluate soil acidity, which directly influences nutrient availability and plant health, ultimately impacting agricultural productivity.
The pH meter definition highlights the paramount significance of pH measurement in environmental contexts. It aids in identifying changes that could disrupt microbial activity and nutrient absorption, both of which are vital for sustaining ecosystem balance. Moreover, pH instruments play a crucial role in the food and beverage industry, ensuring that products meet safety standards by verifying suitable acidity levels to inhibit pathogen growth.
With technological advancements, modern pH devices, including IoT-enabled sensors, are revolutionizing data collection and monitoring capabilities, facilitating real-time analysis and enhancing decision-making in environmental management. This evolution underscores the growing importance of pH devices in safeguarding public health and preserving environmental integrity. Notably, the pH meter definition indicates that the pH sensors market reached an estimated value of USD 1.18 Billion in 2024 and is projected to expand to USD 2.17 Billion by 2034, highlighting the increasing relevance of pH meters across various applications, particularly in environmental monitoring. The durability of lab-grade pH probes against strong acids and bases further underscores their suitability for diverse scientific applications.
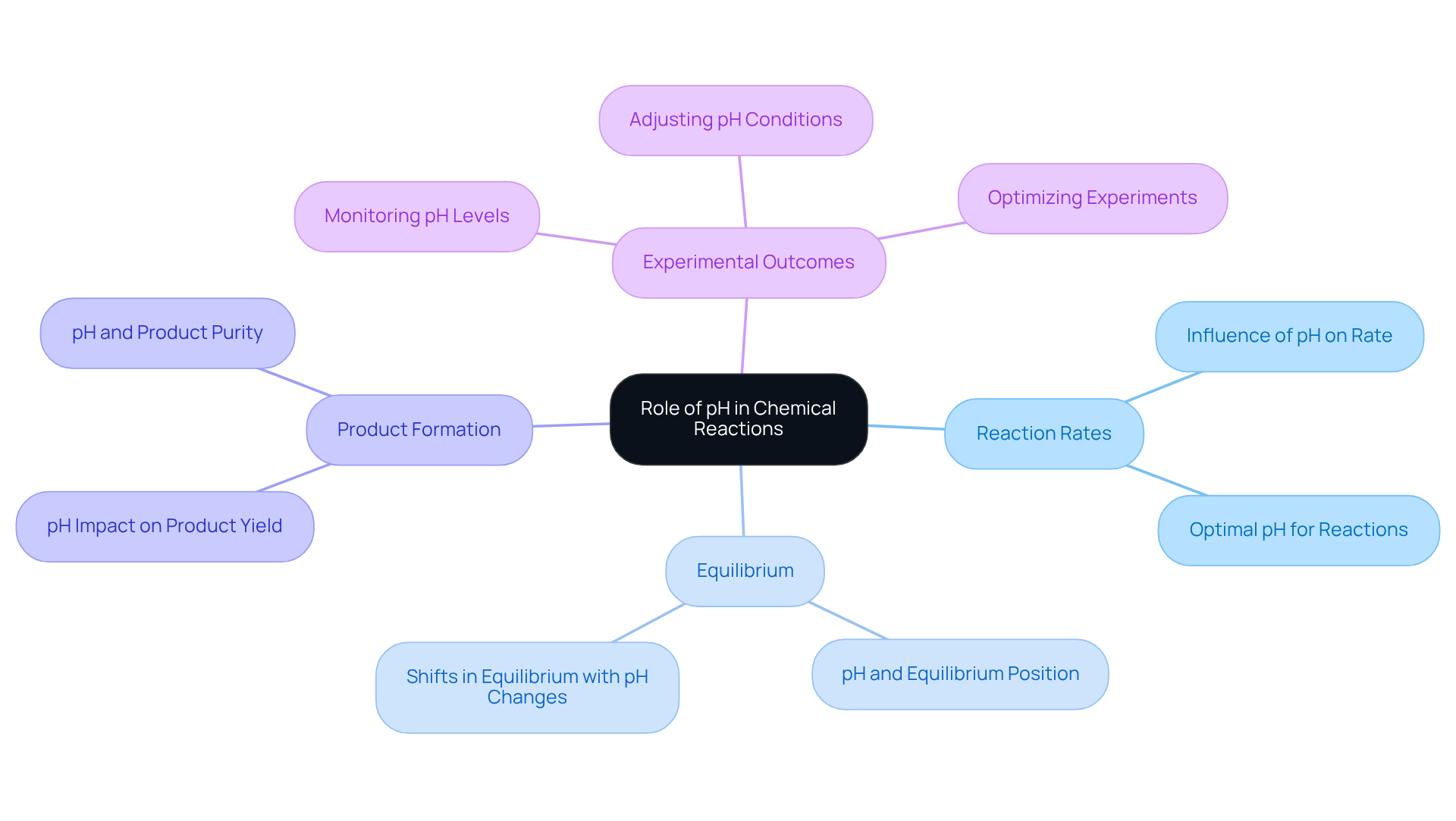
The Role of pH in Chemical Reactions: Implications for Lab Work
pH plays a critical role in chemical reactions, significantly influencing reaction rates, equilibrium, and product formation. It is essential for lab managers to comprehend how variations in pH can impact experimental outcomes, especially in sensitive reactions. By diligently monitoring and adjusting pH levels, research facilities can optimize conditions for successful experiments. This proactive approach leads to more reliable and reproducible results, reinforcing the importance of pH management in laboratory settings.
Reference Electrolyte: Key to Reliable pH Measurements
The reference electrolyte is crucial for the precise operation of pH instruments, providing a stable reference point for accurate measurements. pH is assessed on a scale from 0 to 14, and comprehending this range is vital for lab managers. To ensure precise pH readings, lab managers must prioritize the maintenance and timely replacement of reference electrolytes. Failure to do so can result in significant discrepancies, as the potential difference between the sensing and reference electrodes directly impacts the pH output.
The theoretical potential at pH 7 is 0 mV, with a slope of approximately 59 mV per pH unit change, as articulated by the Nernst equation:
E = E - 2.3 (RT/nF) log a
By recognizing the essential role of reference electrolytes, research facilities can assure consistent and reliable outcomes in their analyses. To maintain precision, lab supervisors should regularly assess the condition of reference electrolytes and replace them as needed, ensuring the optimal performance of their pH devices.
Benefits of Advanced pH Meters: Enhancing Laboratory Efficiency
Sophisticated pH devices, which are explained in the pH meter definition, present a multitude of benefits that significantly enhance efficiency in research environments. With features such as superior accuracy, rapid response times, and intuitive user interfaces, these devices streamline the data collection process, facilitating quicker analysis.
As Dr. Jane Smith, a chemist, aptly remarks, 'In the intricate dance of acid-base reactions, precision according to the pH meter definition illuminates the path to clarity.'
Furthermore, many advanced models come equipped with data logging capabilities and connectivity options, which not only refine record-keeping but also ensure adherence to stringent regulatory standards.
For instance, facilities utilizing sophisticated pH devices have reported marked improvements in their data gathering and analysis processes, leading to more reliable outcomes. A notable case is a pharmaceutical laboratory that integrated advanced pH meter definition into its operations, achieving a remarkable 30% reduction in analysis time.
By investing in these cutting-edge instruments, laboratory managers can enhance their operational capabilities, ultimately cultivating a more productive and compliant laboratory environment.
Conclusion
Understanding the intricacies of pH meters is essential for lab managers aiming to enhance their laboratory's precision and efficiency. This article underscores the pivotal role these instruments play in accurately measuring acidity and alkalinity, which is crucial for a myriad of scientific applications, from pharmaceuticals to environmental monitoring. A comprehensive overview of the features, maintenance practices, and calibration procedures associated with pH meters reveals that investing in high-quality devices, such as those from JM Science, is paramount for achieving reliable results.
Key insights indicate that:
- Regular calibration and diligent maintenance of pH meters—particularly glass electrodes and reference electrolytes—are fundamental for ensuring accuracy in measurements.
- It is crucial to select the appropriate type of pH meter based on specific laboratory needs, whether for fieldwork or controlled environments.
By adhering to best practices in pH measurement, laboratory managers can significantly improve the quality of their research and ensure compliance with regulatory standards.
Ultimately, the significance of pH meters extends beyond mere measurement; they are integral to the reliability of experimental outcomes and the advancement of scientific knowledge. As technology continues to evolve, embracing advanced pH devices will not only streamline laboratory processes but also enhance the overall integrity of scientific work. Lab managers are encouraged to prioritize the adoption of these essential instruments and their proper upkeep to foster a culture of precision and excellence in their research endeavors.




