Overview
This article delves into the critical applications of high-performance liquid chromatography (HPLC) within pharmaceutical development and quality control. HPLC is indispensable for ensuring drug safety and efficacy, playing vital roles in:
- Formulation analysis
- Quality assurance
- Stability testing
- Regulatory compliance
The integration of advanced technologies further enhances the precision and efficiency of pharmaceutical processes. By understanding these applications, professionals can appreciate the importance of HPLC in maintaining high-quality standards in the industry.
Introduction
High-performance liquid chromatography (HPLC) has emerged as a cornerstone in the pharmaceutical industry, revolutionizing the methodologies employed in drug development and quality control. This powerful analytical technique not only guarantees the safety and efficacy of medications but also enhances the precision of formulation processes. As the pharmaceutical landscape evolves, a pivotal question arises: how can HPLC be leveraged to meet the ever-increasing demands for regulatory compliance and innovation? This article delves into eight critical uses of HPLC, exploring its vital role in pharmaceutical development and the advancements that are shaping its future.
JM Science HPLC Solutions: Tailored Instruments for Pharmaceutical Development
JM Science Inc. offers a comprehensive array of HPLC instruments meticulously crafted for pharmaceutical development, including the Hiranuma Aquacounter AQV-300 Volumetric and AQ-300 Coulometric Karl Fischer Titrators. These titrators are indispensable for pharmaceutical and medicine testing, particularly in compliance with the Japanese Pharmacopoeia, guaranteeing precision in moisture content analysis.
The systems provided by JM Science for high-performance liquid chromatography, or HPLC uses, assure accuracy in both formulation and analysis. Equipped with state-of-the-art features, these instruments facilitate the separation and quantification of active medicinal ingredients (APIs) and impurities, which are vital for ensuring safety and efficacy.
By leveraging JM Science's tailored solutions that include HPLC uses and Karl Fischer titration technology, companies within the healthcare sector can enhance their research capabilities and streamline their development processes, ultimately leading to more effective therapeutic offerings.
HPLC in Drug Formulation: Ensuring Quality and Efficacy
HPLC uses are a cornerstone of formulation processes, capturing the attention of researchers and professionals alike. This analytical technique enables the analysis of stability, solubility, and compatibility of various ingredients within a formulation. By utilizing HPLC uses, scientists in the pharmaceutical sector can ascertain that the final product adheres to essential quality standards and therapeutic effectiveness. This method not only identifies optimal formulations that enhance drug delivery but also improves patient outcomes, solidifying its role as a foundational element in drug development.
Quality Control in Pharmaceuticals: The Role of HPLC
HPLC uses stand as a cornerstone in the quality assurance of pharmaceutical products. The HPLC uses of this advanced analytical technique are pivotal for confirming the purity and concentration of active pharmaceutical ingredients (APIs) and finished products, ensuring they meet stringent regulatory standards. HPLC uses effective identification of impurities and degradation products to empower manufacturers to uphold product integrity and safety. Such capabilities are indispensable in mitigating potential health risks linked to contaminated or substandard medications, thereby safeguarding patient welfare and preserving the manufacturer’s reputation.
Pharmacokinetics Studies: Utilizing HPLC for Drug Absorption Analysis
HPLC uses are fundamental in pharmacokinetics, particularly for assessing medication absorption, distribution, metabolism, and excretion. HPLC uses quantification of substance levels in biological fluids such as blood and urine to provide essential insights into a compound's pharmacological effects and therapeutic range. This information is vital for optimizing dosing regimens and ensuring patient safety throughout clinical trials.
Recent studies have demonstrated the efficacy of HPLC uses in evaluating the absorption characteristics of various medications, including antimalarials like quinine, where innovative formulations have shown enhanced bioavailability. For instance, research on nanoparticle formulations has revealed improved medication release and permeation, underscoring the technique's critical role in assessing these advancements. Pharmacologists emphasize that the HPLC uses are indispensable for understanding how substances behave within the body, thereby facilitating the development of safer and more effective treatments.
Moreover, clinical trials that utilize HPLC uses for chromatography data have successfully evaluated drug absorption, leading to significant progress in treatment protocols and patient outcomes. The continued exploration and application of HPLC uses will undoubtedly contribute to the evolution of pharmacokinetic studies and enhance therapeutic strategies.
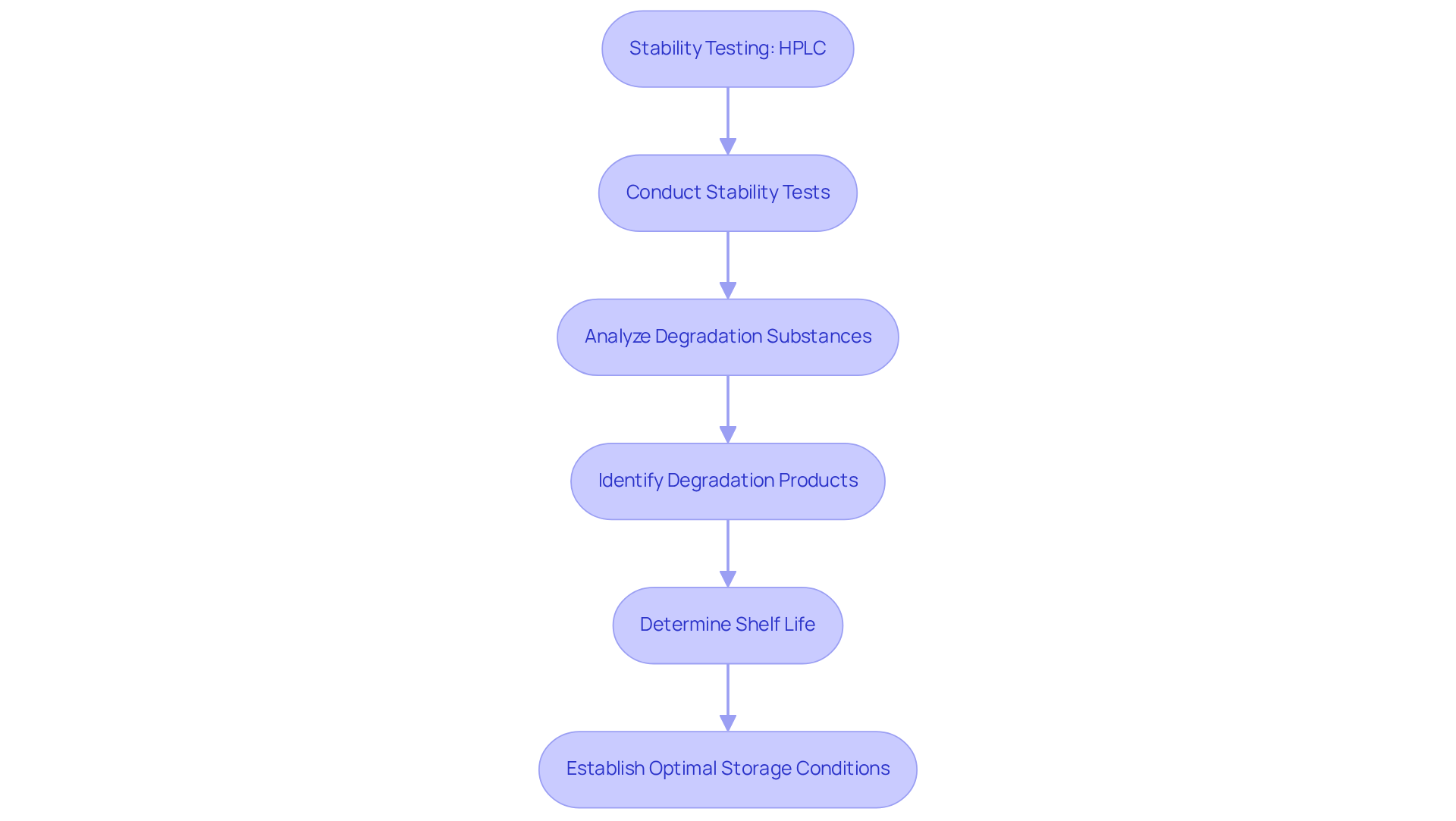
Stability Testing: HPLC's Role in Ensuring Drug Longevity
The hplc uses are essential for conducting stability testing of pharmaceutical products. This technique plays a pivotal role in analyzing degradation substances generated under varying environmental conditions, such as temperature and humidity. By accurately identifying these degradation products, researchers can ascertain the shelf life and optimal storage conditions for medications. Such information is crucial for guaranteeing that medications retain their efficacy and safety throughout their intended usage period. The implications of HPLC uses extend beyond mere analysis; they are foundational to the integrity of pharmaceutical development and patient care.
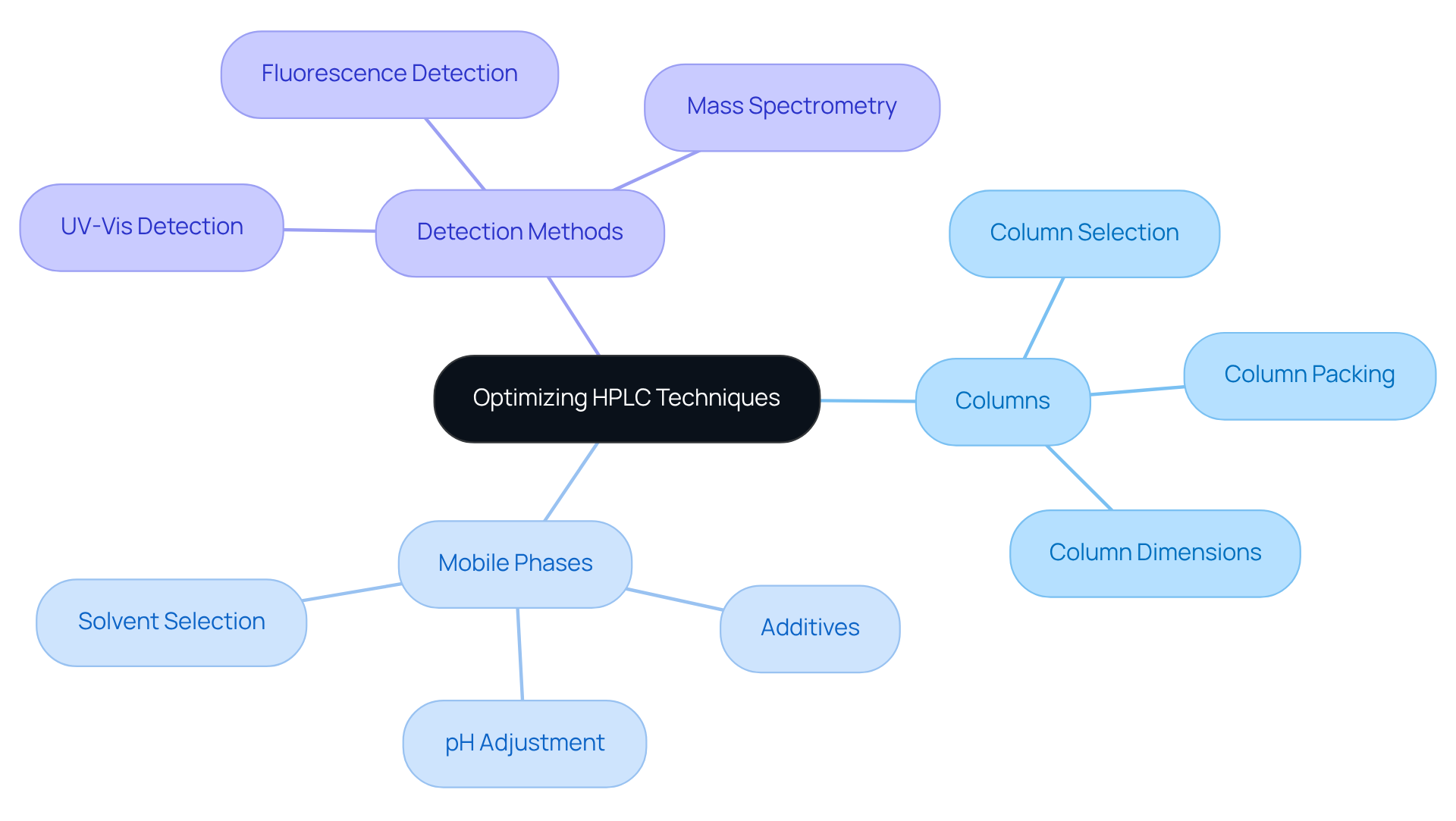
Method Development in Pharmaceuticals: Optimizing HPLC Techniques
Enhancing chromatographic techniques is essential for effective method development in pharmaceuticals. This critical process encompasses the careful selection of:
- Columns
- Mobile phases
- Detection methods
All aimed at achieving optimal separation and quantification of analytes. By employing systematic approaches to technique development, scientists can significantly enhance the consistency and repeatability of chromatography analyses. This ultimately leads to more precise outcomes and superior formulations, reinforcing the importance of rigorous methodologies in the field.
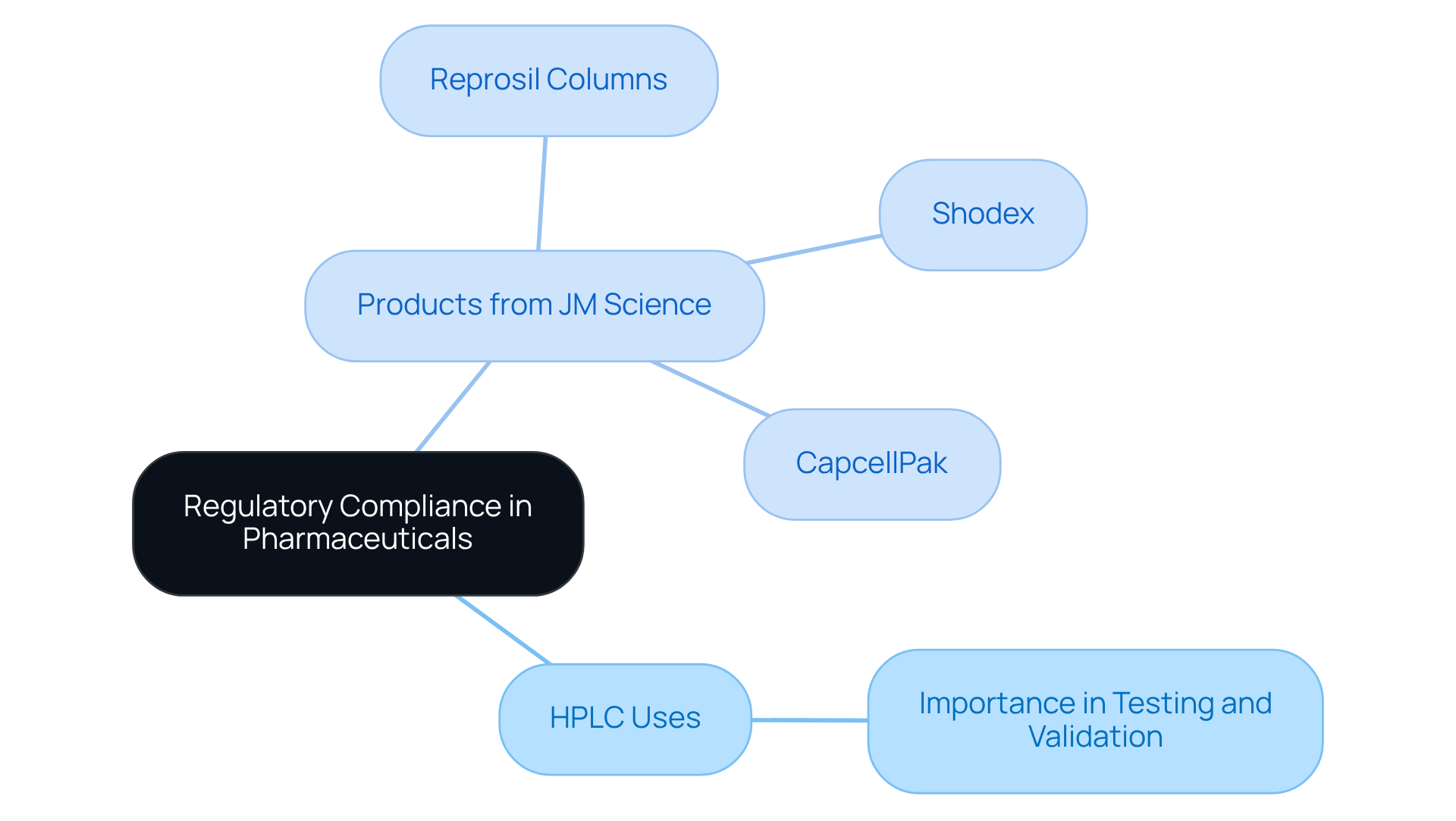
Regulatory Compliance: HPLC's Essential Role in Pharmaceutical Standards
The pharmaceutical industry relies on HPLC uses to ensure regulatory compliance. Regulatory agencies, including the FDA, mandate thorough testing and validation of analytical methods utilized in drug development and quality control. JM Science Inc. provides a comprehensive range of premium liquid chromatography solutions, featuring:
- Shodex
- CapcellPak
- Reprosil columns
Additionally, JM Science offers fittings and accessories, all designed to deliver the precision and accuracy required to meet these stringent standards. By integrating JM Science's advanced chromatography systems, companies can ensure their pharmaceutical products are safe, effective, and of superior quality.
Adhering to chromatography protocols that incorporate HPLC uses with these innovative instruments facilitates smoother regulatory submissions and supports a robust market position. Explore our offerings today to elevate your laboratory's capabilities!
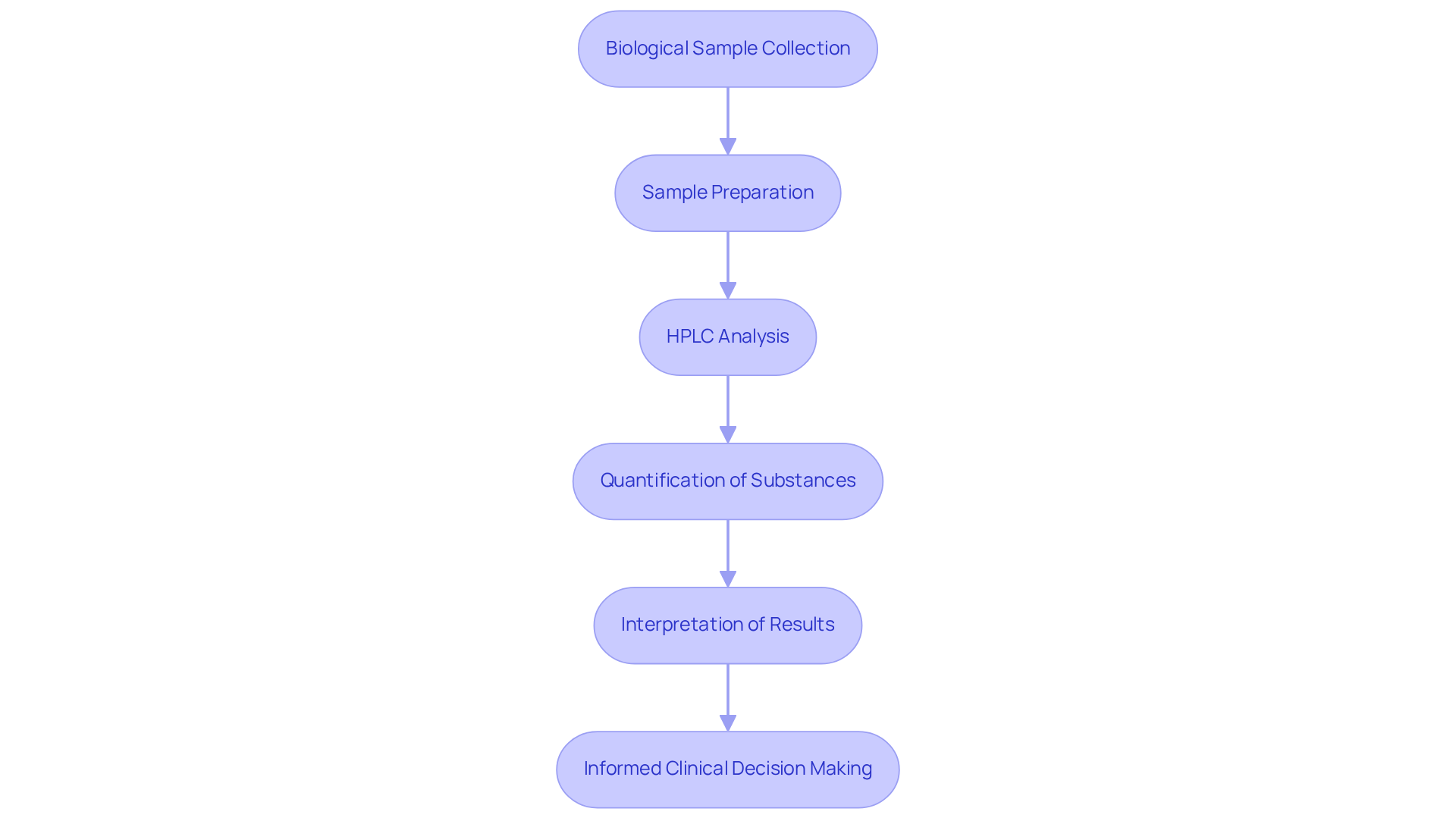
Bioanalysis in Pharmaceuticals: Leveraging HPLC for Drug Effect Evaluation
HPLC uses a pivotal instrument in the realm of bioanalysis, playing a crucial role in evaluating medication effects within clinical settings. HPLC uses meticulous analysis of biological samples, including plasma and urine, to enable the quantification of substance concentrations and metabolites. This process yields invaluable insights into a compound's pharmacokinetics and pharmacodynamics, which are essential for assessing therapeutic efficacy, safety, and potential side effects. Such data not only informs clinical decision-making but also significantly enhances patient outcomes. Ultimately, the HPLC uses in clinical environments underscore its importance as a high-quality scientific instrument, guiding healthcare professionals in their pursuit of optimal patient care.
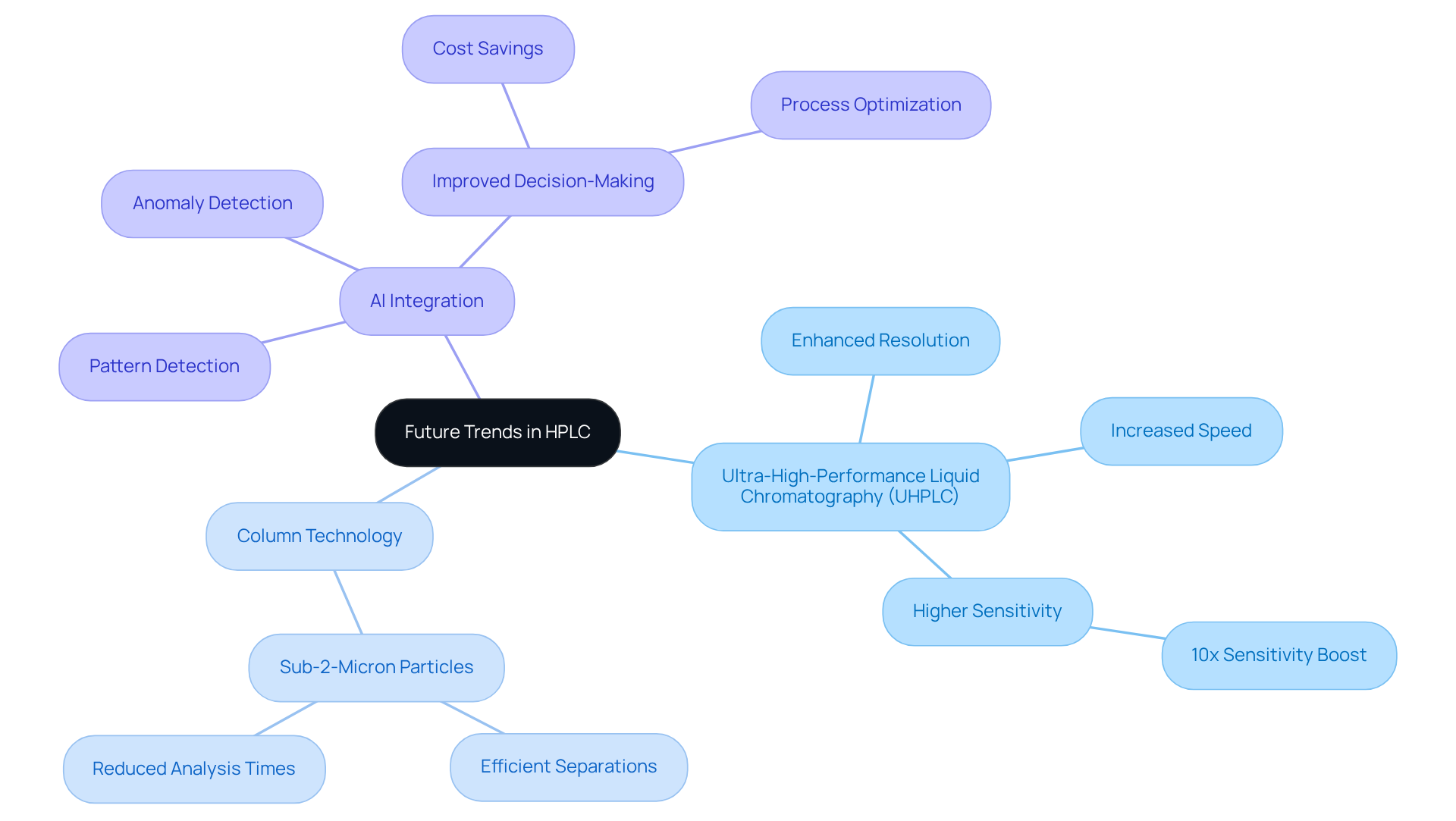
Future Trends in HPLC: Innovations Shaping Pharmaceutical Development
The future of high-efficiency liquid chromatography (HPLC) uses remarkable advancements, primarily fueled by technological innovations and the evolving needs of the pharmaceutical sector. A notable trend is the emergence of ultra-high-performance liquid chromatography (UHPLC), which dramatically enhances resolution and speed, showcasing its HPLC uses by facilitating quicker analyses and greater throughput in pharmaceutical development. Recent studies indicate that HPLC uses can boost sensitivity by as much as 10 times compared to traditional high-performance liquid chromatography systems, revolutionizing the detection of low-concentration compounds critical for pharmaceutical research.
Innovations in column technology are equally crucial, with the advent of sub-2-micron particles that enable more efficient separations and reduce analysis times. This progress not only accelerates the drug development process but also enhances the accuracy of quality control measures, with HPLC uses helping to ensure that pharmaceutical products comply with stringent regulatory standards. JM Science Inc. offers a diverse range of HPLC columns, including Shodex and CapcellPak, that are specifically designed to optimize HPLC uses and advancements.
Furthermore, the integration of artificial intelligence (AI) in data analysis is transforming how laboratories interpret complex chromatographic data. AI algorithms can detect patterns and anomalies in data sets, resulting in faster decision-making and improved process optimization. For example, AstraZeneca's analytical scientists have harnessed AI to refine their testing methods, leading to substantial cost savings and enhanced product quality.
As these innovations continue to progress, they are poised to redefine the landscape of medicine development, driving efficiency and effectiveness in drug discovery processes, especially with HPLC uses in quality assurance. JM Science Inc. is pivotal in this evolution, offering premium HPLC systems, columns, and accessories at competitive prices, ensuring that pharmaceutical lab managers have access to the advanced tools essential for success in their vital work.
Conclusion
The significance of high-performance liquid chromatography (HPLC) in pharmaceutical development and quality control is paramount. This advanced analytical technique is essential for ensuring the safety, efficacy, and regulatory compliance of pharmaceutical products. By employing HPLC, researchers and manufacturers can accurately assess the composition of drug formulations, confirm the purity of active ingredients, and evaluate the stability of medications over time.
This article highlights several key applications of HPLC, including:
- Drug formulation
- Quality assurance
- Pharmacokinetics studies
- Stability testing
- Method development
- Regulatory compliance
Each of these areas underscores how HPLC contributes to optimizing drug delivery, enhancing patient outcomes, and ensuring that products meet stringent industry standards. Furthermore, advancements in HPLC technology, such as ultra-high-performance liquid chromatography and AI integration, promise to revolutionize the field, making analyses faster and more precise.
As the pharmaceutical landscape continues to evolve, the importance of HPLC will only grow. Companies are encouraged to adopt these cutting-edge technologies and methodologies to enhance their research and development capabilities. By leveraging HPLC solutions, organizations can not only improve their product offerings but also uphold their commitment to patient safety and regulatory standards. Embracing these innovations will pave the way for more effective and reliable therapeutic solutions, ultimately benefiting the healthcare industry and the patients it serves.




