Overview
Best practices for clinical laboratory (CL) measurement are essential for ensuring accuracy and compliance. This is achieved through rigorous calibration, effective quality control, and strict adherence to regulatory standards. Implementing these practices not only enhances measurement reliability but also significantly improves patient care outcomes.
Furthermore, leveraging advanced technology and committing to continuous training are critical components that bolster the effectiveness of these practices. By focusing on these key areas, laboratories can elevate their operational standards and ultimately deliver better healthcare services.
Introduction
In the realm of healthcare, the precision of clinical laboratory (CL) measurements transcends mere technical requirement; it stands as a critical factor influencing patient outcomes and treatment decisions.
As laboratories navigate the complexities of diagnosing and monitoring health conditions, the significance of accurate measurement protocols becomes increasingly apparent.
Recent studies have revealed alarming discrepancies in quality measures across health systems, amplifying the urgency for enhanced accuracy.
This article delves into the significance of CL measurement, explores best practices for achieving accuracy, and examines the regulatory frameworks ensuring laboratories uphold the highest standards.
By leveraging advanced technologies and implementing effective strategies, clinical laboratories can significantly improve their measurement practices, ultimately leading to better patient care and enhanced operational efficiency.
Understanding CL Measurement: Definition and Importance
Clinical Laboratory (CL) evaluation is fundamental, encompassing the quantitative assessment of various analytes in biological samples. This process serves as a cornerstone for diagnosing and monitoring health conditions. The precision of these evaluations is paramount, as they directly influence clinical decisions, treatment plans, and ultimately, patient outcomes. Recent studies reveal a mean rate of quality measure discrepancies in health systems at 16.5%, with ambulatory practices showing a rate of 11.2%. Notably, some facilities have reported measure discrepancy rates exceeding 20%. Such discrepancies can significantly affect performance rates, underscoring the necessity for accurate evaluation protocols in clinical laboratories.
The importance of accurate CL measurement cannot be overstated; it is essential for maintaining high standards of reliability and integrity in both patient care and research. For example, although the use of albumin-adjusted calcium is frequent in clinical settings, its limited reliability has been called into question. This situation highlights the need for clinicians to critically evaluate routine practices to avoid misclassification and unnecessary complexity in patient management.
Expert opinions further emphasize the significance of CL evaluation in healthcare. As noted by John D D’Amore, President & Head of Informatics, "This research demonstrates that discrepancies affect performance rates for some, but not all, quality measures." This statement underscores the critical nature of precise evaluations, as discrepancies can lead to varying performance rates across quality measures, ultimately impacting patient care.
Furthermore, case studies have illustrated that employing standardized metrics, such as standard scores and percentile rank scores in psychological assessments, can enhance the clarity and applicability of test results across diverse populations. Real-world examples demonstrate the profound impact of precise CL measurement on patient outcomes. Facilities that have adopted strict assessment standards report discrepancies below 5%, showcasing the advantages of accuracy in testing practices.
As the healthcare environment evolves, the commitment to enhancing CL measurement accuracy remains a crucial emphasis for facilities. This dedication ensures that they meet the needs of effective patient care and contribute to advancements in medical science.
Techniques for Accurate CL Measurement: Best Practices
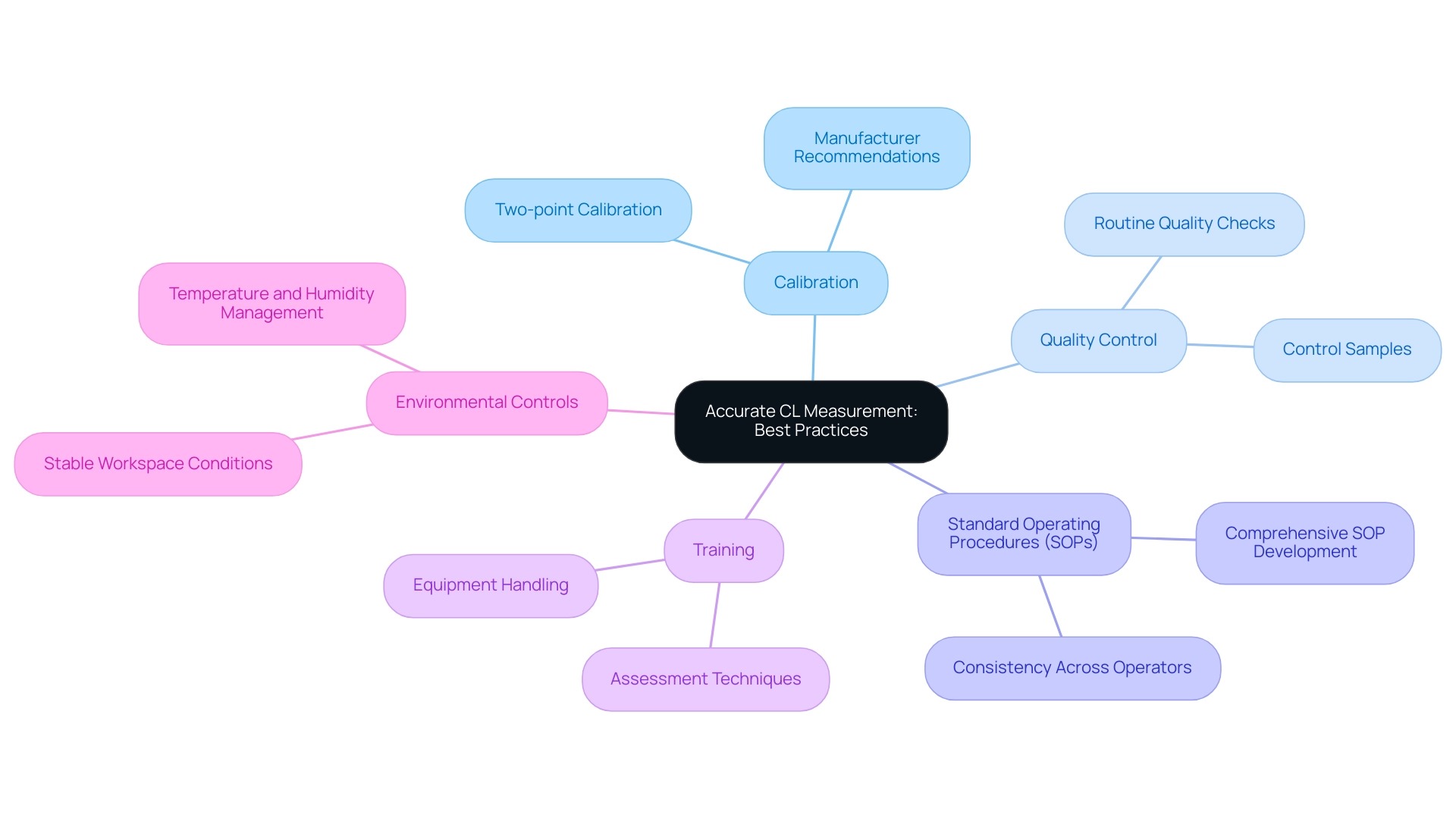
To achieve accurate chloride (CL) measurements, laboratories must adopt several best practices that are essential for precision and reliability.
- Calibration is a foundational step. Regular calibration of instruments using certified reference materials is crucial. The frequency of calibration should be set at a minimum of two-point calibration after any reagent lot change and whenever quality control procedures necessitate it. As noted by David Mullins, "Good Management! When you open the book that came with the equipment, what is the manufacturers recommendation on calibration frequency? Determining this frequency yourself can be an expensive and time-consuming operation." This approach minimizes the risk of inaccuracies that can arise over time.
- Quality Control is another critical component. Implementing routine quality control checks with control samples is vital for monitoring instrument performance. These checks help identify any deviations from expected results, allowing for timely corrective actions that uphold measurement integrity.
- Next, Standard Operating Procedures (SOPs) play a significant role in minimizing variability. Developing and adhering to comprehensive SOPs for all assessment processes is essential. SOPs should outline each step of the evaluation process, ensuring consistency and reliability across different operators and conditions.
- Training is fundamental to the success of these practices. Ensuring that all personnel are adequately instructed in assessment techniques and equipment handling enhances the precision of assessments and reduces the likelihood of errors during the testing process.
- Finally, Environmental Controls must not be overlooked. Maintaining stable conditions within the workspace, such as temperature and humidity, is necessary to mitigate external influences on readings. Variations in the research setting can significantly affect the precision of CL readings.
Together, these methods enhance the dependability of CL measurement assessments, ultimately improving the quality of test outcomes. The statistics indicate that calibration frequency is set at two-point calibration after reagent lot changes and when quality control procedures require it, underscoring the importance of adhering to these guidelines. Additionally, JM Science Inc. offers comprehensive customer assistance and resources, including instructional videos and application libraries, to help facilities adopt these optimal methods efficiently.
By prioritizing these best practices, facilities can enhance measurement accuracy and reduce troubleshooting costs, fostering a culture of quality and precision in clinical analysis.
Regulatory Compliance in CL Measurement: Key Standards and Guidelines
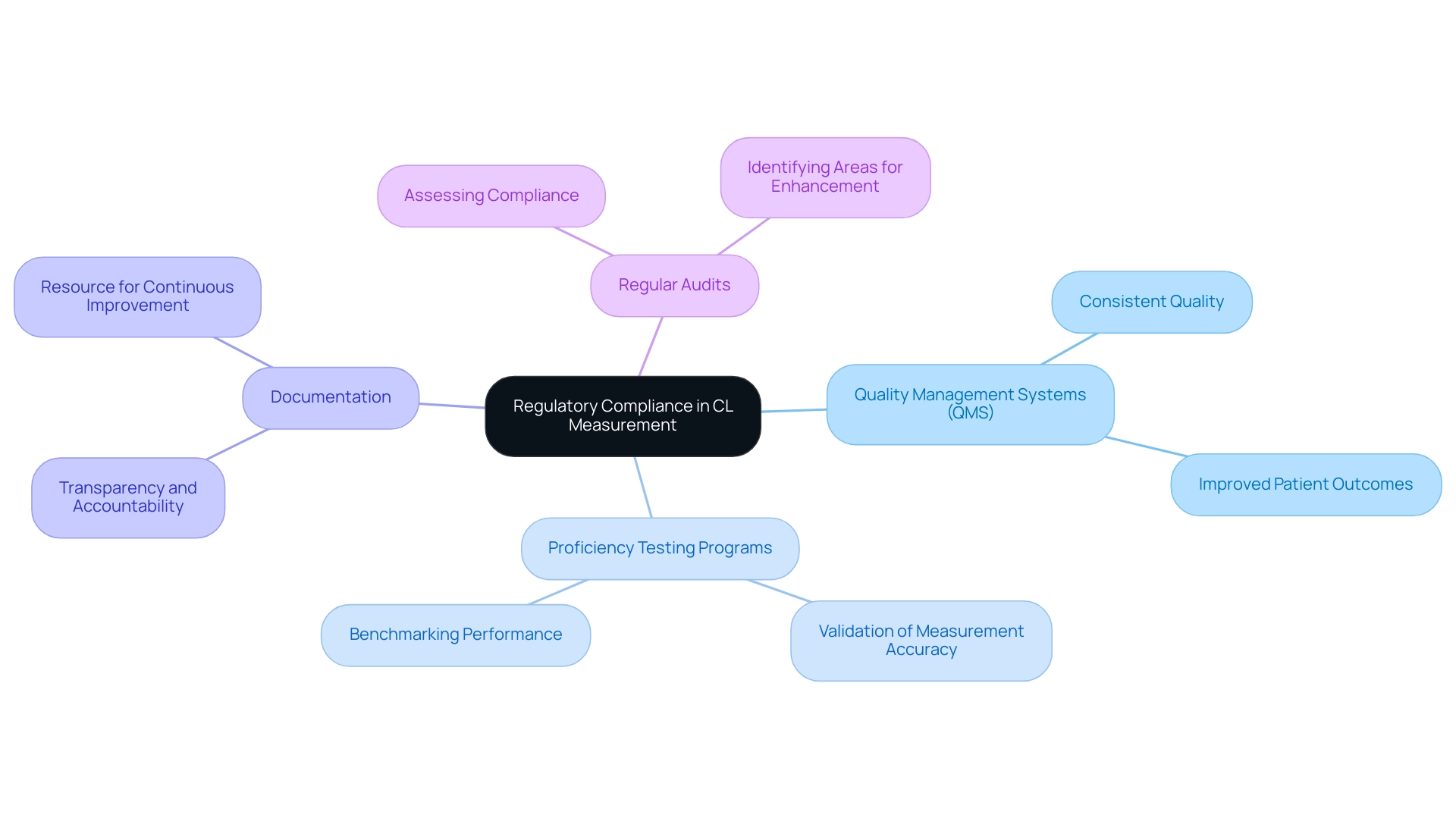
Adherence to regulatory standards, such as the Clinical Laboratory Improvement Amendments (CLIA), is essential for institutions to operate both legally and ethically. Compliance with these standards not only enhances credibility but also plays a vital role in safeguarding patient health. Key standards include:
- Quality Management Systems (QMS): Establishing a robust QMS is crucial for maintaining consistent quality throughout operations. A well-structured QMS facilitates adherence to protocols and enhances overall performance, leading to improved patient outcomes.
- Proficiency testing programs are critical for validating CL measurement accuracy. These programs assist facilities in benchmarking their performance against established standards for CL measurement, ensuring that results are reliable and trustworthy.
- Documentation: Comprehensive documentation of all procedures, results, and quality control measures is necessary for maintaining transparency and accountability. This practice not only aids in compliance but also serves as a valuable resource for continuous improvement.
- Regular Audits: Conducting internal audits is vital for assessing compliance with established protocols. These audits help identify areas for enhancement and ensure that facilities consistently meet regulatory requirements.
Recent statistics indicate that facilities achieving compliance with CLIA standards experience a significant reduction in turnover intentions among professionals, attributed to a heightened sense of procedural and distributive justice within the workplace. This correlation underscores the importance of nurturing a fair and supportive atmosphere, which can lead to improved job retention and overall performance. The study titled "The Role of Justice Theory in Laboratory Professional Dynamics" highlights how higher perceptions of procedural and distributive justice are associated with a positive professional identity, ultimately reducing turnover intentions.
As facilities prepare for the 2025 updates to CLIA compliance, including acceptance limits for Uric Acid set at TV ± 17%, it is imperative to stay informed about evolving regulatory standards and implement effective Quality Management Systems. By doing so, facilities can not only meet compliance requirements but also enhance their operational efficiency and contribute positively to patient care. As Ali B Mahmoud noted, the study received ethics approval from the Drexel University Office of Research & Innovation Institutional Review Board, emphasizing the significance of ethical considerations in research practices.
JM Science's commitment to consistently refreshing its product range and sustaining robust connections with leading manufacturers further assists facilities in attaining compliance and improving quality management systems.
Challenges in CL Measurement: Overcoming Common Obstacles
Clinical laboratories frequently face a range of challenges in chloride (CL) testing that can impact the precision and reliability of results. Key issues include instrument variability, sample handling errors, regulatory changes, and data management.
Instrument variability is a significant concern, as variations in instrument performance can lead to inconsistent results. Regular calibration and maintenance of equipment are essential to mitigate this variability and ensure consistent outcomes across different testing scenarios. The significance of dependable instrumentation is underscored by JM Science's commitment to providing high-quality scientific tools, including high-performance liquid chromatography (HPLC) solutions, titrators, Karl Fischer reagents, and a diverse selection of HPLC columns and accessories, all designed to enhance accuracy and reliability.
Sample handling errors can also severely compromise the integrity of results. For an FDA-approved test method, it is ideal to test 40 healthy samples (20 men and 20 women) to ensure representative sampling. To minimize these errors and improve overall measurement reliability, implementing comprehensive training programs for technical staff on proper sample collection and handling techniques is crucial.
Moreover, the ever-evolving landscape of testing regulations can present compliance challenges. Establishing a dedicated compliance team to monitor regulatory updates and revise protocols accordingly can help laboratories stay ahead of these changes and maintain adherence to standards.
Data management is another critical area, as handling large volumes of data can lead to potential errors in analysis and reporting. Utilizing Laboratory Information Management Systems (LIMS) can streamline data handling processes, thereby reducing the likelihood of errors and enhancing overall efficiency.
Proactively addressing these challenges is vital for enhancing the reliability of CL measurement. A recent case study on method comparison highlighted the importance of evaluating analytical methods to ensure accuracy. It emphasized the necessity of comprehensive statistical analysis, such as Pearson’s correlation coefficient and regression analysis, to identify differences between methods, thereby reinforcing the validity of diagnostic results.
Expert insights further indicate that maintaining high standards in testing practices is essential for overcoming instrument variability. Dr. Vivek Pant, a consultant biochemist, notes, "In this article, the basic statistics is explained in its simplest form," emphasizing the importance of clarity in protocols, particularly in addressing instrument variability. Additionally, the Multiple of the Median (Mom) is utilized in screening tests to present results, normalizing findings based on median values from unaffected groups, which is especially significant in the context of CL assessment.
By applying these strategies and leveraging JM Science's innovative medical devices, such as electronic stethoscopes that facilitate remote patient monitoring, facilities can significantly enhance their evaluation precision and adherence, ultimately leading to improved patient outcomes. Explore JM Science's extensive selection of products to elevate your research methods today!
Leveraging Technology for Enhanced CL Measurement Accuracy
Technological advancements play a crucial role in enhancing the accuracy of CL measurement. The integration of innovative technologies has transformed research practices, leading to improved precision and reliability. Key technologies include:
- Automated Analyzers: These advanced instruments significantly reduce human error and enhance throughput, allowing facilities to efficiently manage a greater quantity of samples. A study conducted over three similarly organized days demonstrates that the implementation of total automation can boost productivity while minimizing reliance on manual work, particularly beneficial in environments facing workforce shortages. The study concludes that total automation not only enhances productivity but also decreases the need for manual labor.
- Artificial Intelligence (AI): AI algorithms are revolutionizing the analysis of complex data sets, enabling laboratories to identify patterns that may not be immediately apparent. This capability enhances diagnostic precision, as AI assists in recognizing anomalies and refining decision-making processes in clinical diagnostics.
- Real-time Monitoring Systems: The implementation of systems that continuously observe environmental conditions ensures optimal assessment conditions are maintained. This proactive strategy mitigates factors that could compromise CL measurement precision, thereby enhancing the reliability of results.
- Advanced Calibration Tools: Employing state-of-the-art calibration tools is essential for preserving instrument precision over time. Routine calibration against established standards guarantees that assessment devices remain compliant with CL measurement precision requirements, which is vital for effective patient management.
The significance of CL measurement in assessments is underscored by Shander's assertion that insufficient evaluation correlates with inappropriate transfusion choices, highlighting the critical need for exactness in clinical environments. Additionally, the case study titled "Tolerance Level Analysis for Hemoglobin Assessment" illustrates real-world applications of accuracy improvements, particularly concerning low hemoglobin levels, which are essential for effective patient blood management. By adopting these technologies, facilities can significantly enhance the quality of their CL measurement assessments, ultimately leading to improved patient outcomes and more informed clinical decisions.
The continuous evolution of assessment technologies emphasizes the importance of staying current with innovations to meet the demands of contemporary research methodologies.
Practical Strategies for Implementing Best Practices in CL Measurement
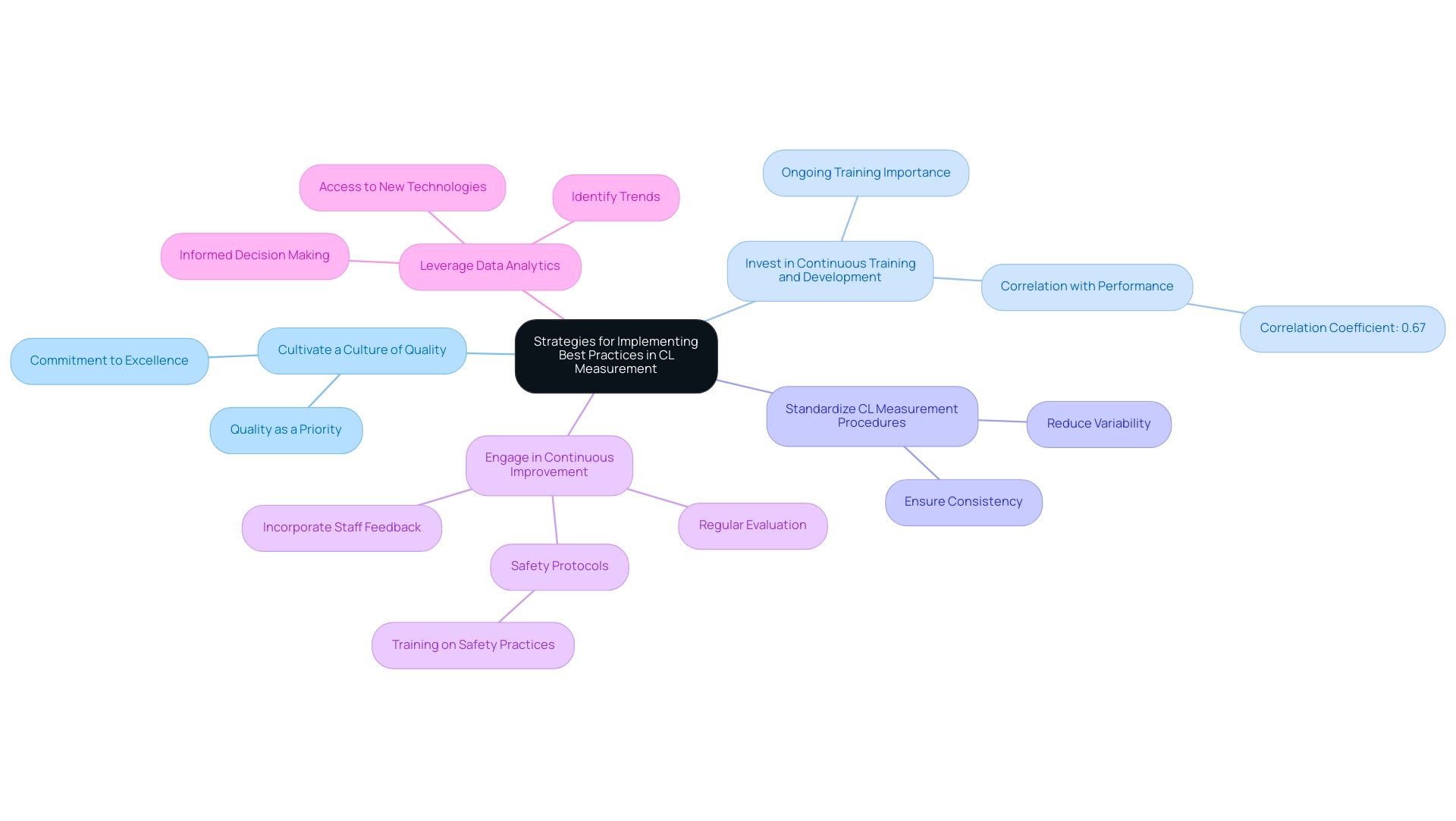
To effectively implement best practices in chloride (CL) measurement, laboratory managers should adopt the following strategies:
- Cultivate a Culture of Quality: Establish an environment where quality is a fundamental priority across all operational activities. This culture not only enhances compliance but also fosters a commitment to excellence among staff.
- Invest in Continuous Training and Development: Ongoing training is essential for keeping laboratory staff updated on the latest best methods and technological advancements. Research indicates that organizations that prioritize training see a significant improvement in operational performance, with a correlation of 0.67 between training investment and integrated clinical care performance. This statistic highlights the significance of investing in staff development to improve evaluation practices.
- Standardize CL Measurement Procedures: Implement standardized protocols for all CL measurement processes to reduce variability and ensure consistency. This standardization is crucial for upholding precision and reliability in CL measurement test outcomes.
- Engage in Continuous Improvement: Regularly evaluate and enhance methods based on staff feedback and emerging research. This proactive strategy not only improves assessment precision but also corresponds with the changing requirements of the scientific community. For instance, establishing safety protocols and training personnel on safety practices, as emphasized in the case study 'Safety Management in Laboratories,' is vital for sustaining a secure environment and promoting a culture of quality that results in enhanced performance.
- Leverage Data Analytics: Utilize data analytics to identify trends and pinpoint areas for enhancement in accuracy. By analyzing performance data, facilities can make informed decisions that drive quality enhancements. JM Science's dedication to refreshing product selections and fostering robust connections with producers backs this strategy, guaranteeing that facilities have access to the newest technologies that improve evaluation methods.
By adopting these strategies, facilities can significantly enhance their assessment practices, ensuring adherence to regulatory standards and improving overall operational quality. As Irwin Z. Rothenberg aptly stated, "These times call for more than good management, they call for good leadership, but leadership that is more adaptive and agile than ever before." This viewpoint underscores the necessity for adaptive leadership in fostering a quality culture within research facilities.
Key Takeaways: Ensuring Accuracy and Compliance in CL Measurement
Ensuring accuracy and compliance in CL measurement is critical for laboratories aiming to enhance patient care and meet regulatory standards. This process begins with a clear understanding of CL measurement and its significance in clinical diagnostics. Applying best practices in evaluation techniques is essential, as it directly influences the reliability of results.
Laboratories must adhere to established regulatory standards, which not only guide operational procedures but also help mitigate common challenges encountered in the evaluation process.
Utilizing technology plays a crucial role in enhancing CL measurement precision. Advanced analytical instruments, such as the high-performance liquid chromatography (HPLC) systems provided by JM Science Inc., leverage CL measurement for the precise quantification of chloride levels, thereby improving the overall quality of test results. JM Science's premium HPLC solutions, alongside their titrators, Karl Fischer reagents, HPLC fittings, and cutting-edge medical devices, are designed to assist facilities in achieving high standards of precision and compliance.
Continuous improvement strategies, including regular training for staff and the integration of quality control measures, further strengthen compliance and accuracy.
The impact of following optimal guidelines in CL assessment is substantial. Research has shown that facilities adopting strict compliance protocols report a 99.2% negative percent agreement in lower prevalence populations, underscoring the importance of precise evaluations in clinical environments. Additionally, case studies demonstrate how specific methodologies, such as comprehensive literature searches, can lead to the identification of reliable evidence for clinical approaches, ultimately enhancing patient outcomes.
The literature search methodology case study exemplifies how a rigorous approach can compile reliable evidence, which is vital for effective clinical decision-making.
Key areas for compliance in CL measurement assessment include:
- Maintaining proper calibration of instruments
- Ensuring the use of validated methods
- Conducting regular audits of testing practices
As noted by Lami YEO, "transvaginal sonographic CL has restricted precision in forecasting spontaneous preterm birth in women with twin pregnancies and threatened preterm labor," highlighting the challenges faced in clinical evaluations. By focusing on these aspects, facilities can not only enhance their testing precision but also ensure adherence to regulatory standards, thereby improving patient care and safety.
Moreover, it is essential to report results for the intended use population separately from other results to prevent misinterpretation, emphasizing the importance of clarity in reporting. In summary, a commitment to best practices in clinical laboratory measurement, supported by JM Science's advanced instruments, including their HPLC systems, Karl Fischer reagents, and innovative medical devices, is crucial for achieving high standards of accuracy and compliance in CL measurement, which are vital for effective patient management.
Conclusion
Accurate clinical laboratory (CL) measurements are not merely a technical necessity; they are essential for ensuring optimal patient outcomes and informed treatment decisions. This article has underscored the critical importance of precision in CL measurements, revealing significant discrepancies across health systems that highlight the urgent need for enhanced accuracy. By implementing best practices such as regular calibration, quality control, and standardized operating procedures, laboratories can substantially reduce variability and improve the reliability of their results.
Moreover, adherence to regulatory standards like the Clinical Laboratory Improvement Amendments (CLIA) is vital for maintaining laboratory credibility and safeguarding patient health. Engaging in proficiency testing and conducting regular audits are fundamental components of a robust quality management system that fosters trust and accountability. The integration of advanced technologies, including automated analyzers and artificial intelligence, further enhances measurement accuracy, enabling laboratories to keep pace with the evolving demands of healthcare.
The challenges faced in CL measurement, including instrument variability and sample handling errors, can be effectively mitigated through proactive strategies and continuous improvement initiatives. By cultivating a culture of quality, investing in personnel training, and leveraging data analytics, laboratories can not only meet compliance requirements but also elevate their operational efficiency.
In conclusion, the commitment to best practices in CL measurement is paramount for enhancing patient care and ensuring regulatory compliance. By prioritizing accuracy and operational excellence, clinical laboratories can significantly contribute to improved health outcomes and advance the field of medical science. As the healthcare landscape continues to evolve, the emphasis on precise measurements will remain critical, making it essential for laboratories to remain informed and adaptive in their practices.




