Overview
Corrosion measurement in pharmaceutical labs is essential for safeguarding equipment integrity and preventing contamination. Techniques such as:
- Electrochemical Impedance Spectroscopy (EIS)
- Linear Polarization Resistance (LPR)
- Ultrasonic Thickness Measurement
stand out as key methods in this regard. These approaches facilitate real-time monitoring and adherence to regulatory standards, which are critical for ensuring safety and operational efficiency in pharmaceutical environments. By employing these methods, labs can not only protect their equipment but also enhance their compliance and reliability. Therefore, it is imperative for pharmaceutical facilities to integrate these corrosion measurement techniques into their operational protocols.
Introduction
Corrosion poses a silent yet significant threat to the integrity of pharmaceutical laboratories. Even the slightest deterioration can lead to catastrophic contamination and costly operational failures. Understanding the various types of corrosion and the methodologies for measuring them is not just beneficial; it is essential for maintaining the reliability of critical equipment. With numerous advanced techniques available, laboratories must ensure they are effectively monitoring and mitigating these risks while adhering to stringent regulatory standards.
Understand Corrosion Measurement Fundamentals
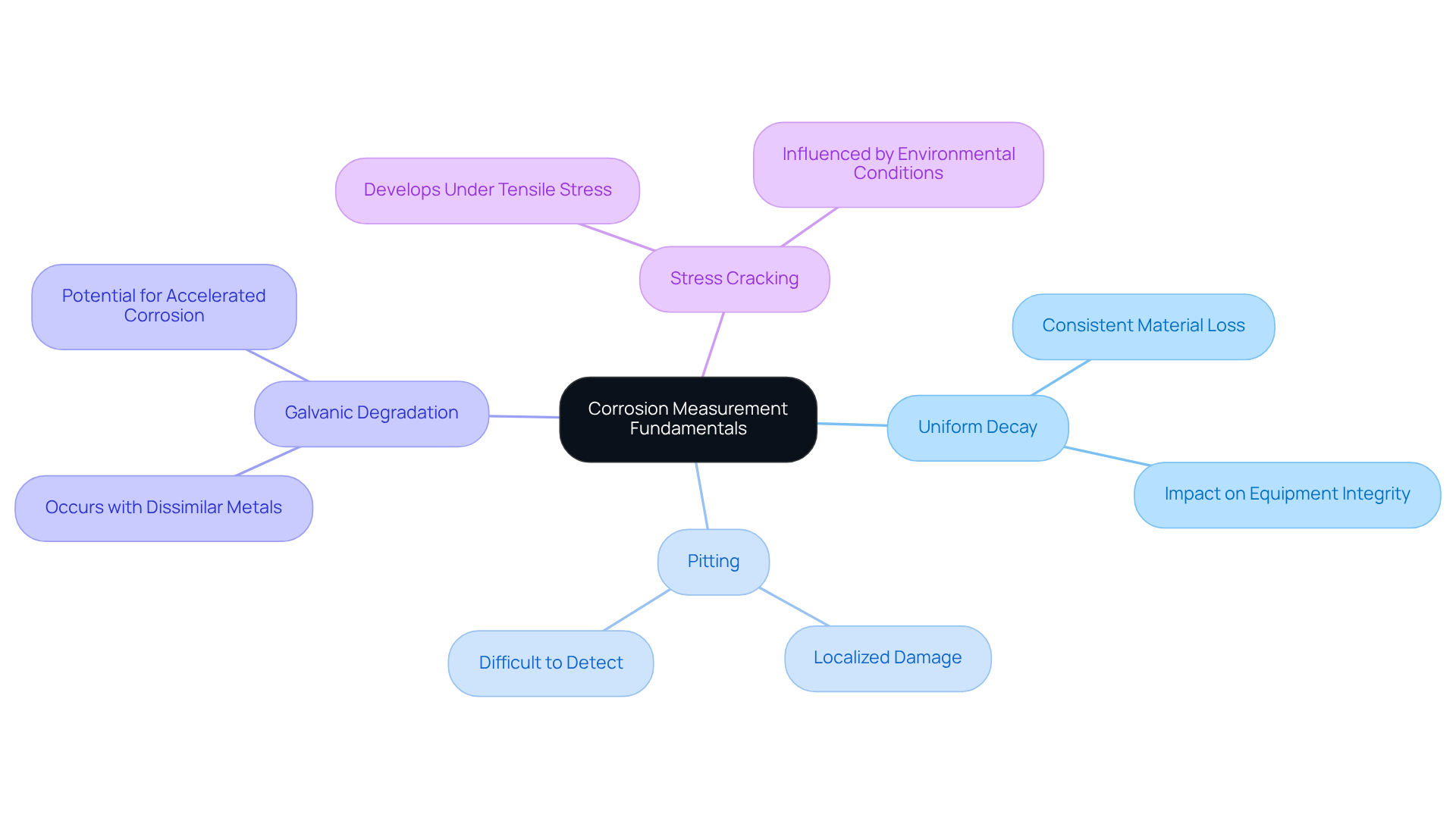
Corrosion, the deterioration of materials—particularly metals—due to chemical reactions with their surroundings—poses a significant risk in pharmaceutical environments. This degradation threatens the integrity of machinery, potentially leading to contamination and flawed results. The primary types of deterioration affecting laboratory equipment include:
- Uniform decay, characterized by a consistent loss of material
- Pitting, which results in localized damage
- Galvanic degradation, which occurs when dissimilar metals are in contact
- Stress cracking, which can develop under tensile stress in corrosive environments
Understanding these types of deterioration is crucial for selecting appropriate methods and substances for corrosion measurement. Research indicates that such deterioration can lead to substantial financial losses in pharmaceutical operations, with estimates suggesting that deterioration-related failures may account for up to 20% of maintenance expenses in industrial settings.
Several factors influence deterioration rates, including environmental conditions like humidity and temperature, material characteristics, and the presence of corrosive substances. For example, a case study involving stainless-steel canisters revealed that chloride salts significantly increased the risk of stress-related cracking, particularly in coastal environments.
Familiarity with these fundamentals is essential for implementing effective assessment strategies in laboratory settings, ensuring the reliability and longevity of critical pharmaceutical equipment.
Implement Advanced Measurement Techniques
Pharmaceutical labs can significantly enhance their monitoring capabilities by utilizing several advanced corrosion measurement techniques.
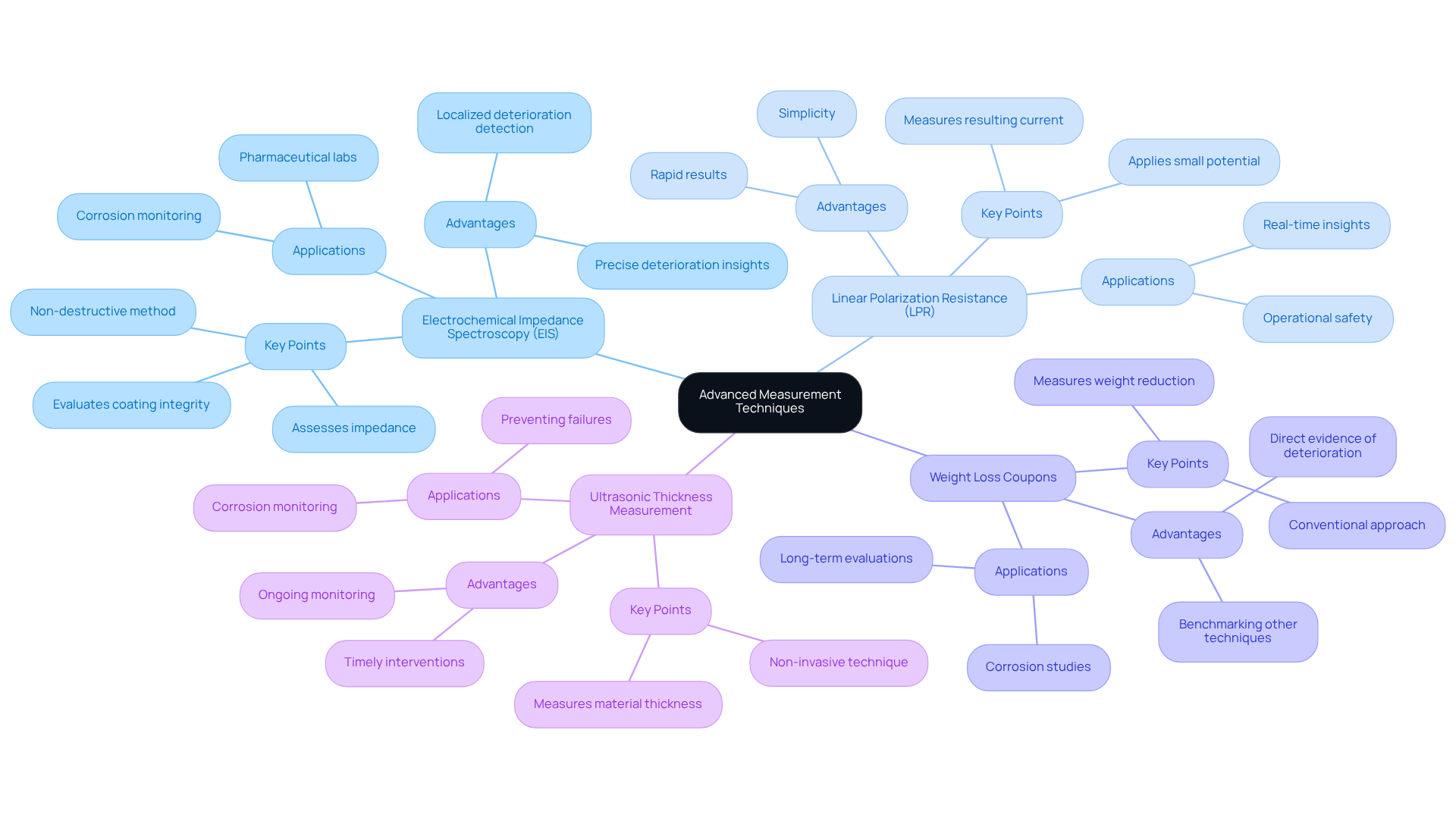
Electrochemical Impedance Spectroscopy (EIS) stands out as a non-destructive method that assesses the impedance of a deteriorating system, offering valuable insights into deterioration rates and mechanisms. This technique is particularly efficient in evaluating the integrity of coatings and detecting localized deterioration, making it a preferred option in settings where precision is paramount. For example, the relationship between shear wave velocity and temperature can greatly influence the accuracy of EIS measurements, as evidenced by various studies.
Linear Polarization Resistance (LPR) is another commonly used technique that involves applying a small potential to the electrode and measuring the resulting current to assess deterioration rates. This method is favored for its simplicity and rapid results, enabling swift evaluations of deterioration conditions. Industry experts assert that LPR can provide real-time insights that are crucial for maintaining operational safety.
Weight Loss Coupons represent a conventional approach, where metal coupons are placed in a corrosive environment, and weight reduction is measured over time to determine deterioration rates. While this technique offers direct evidence of deterioration, it necessitates longer exposure durations and may not capture real-time changes. Nonetheless, case studies have shown that this method can effectively evaluate other techniques, such as ultrasonic assessments.
Ultrasonic Thickness Measurement employs sound waves to measure material thickness, allowing for the identification of deterioration without damaging equipment. This non-invasive technique is particularly advantageous for ongoing monitoring, as it can detect changes in wall thickness over time, thereby preventing potential failures. Recent studies highlight that the average signal filtering process enhances the accuracy of these measurements, facilitating timely interventions.
By implementing these methods, labs can achieve real-time corrosion measurement, which enables prompt interventions and maintenance. The integration of EIS, in particular, bolsters the ability to identify early signs of deterioration, ultimately contributing to safer and more efficient operations within pharmaceutical environments.
Ensure Compliance with Regulatory Standards
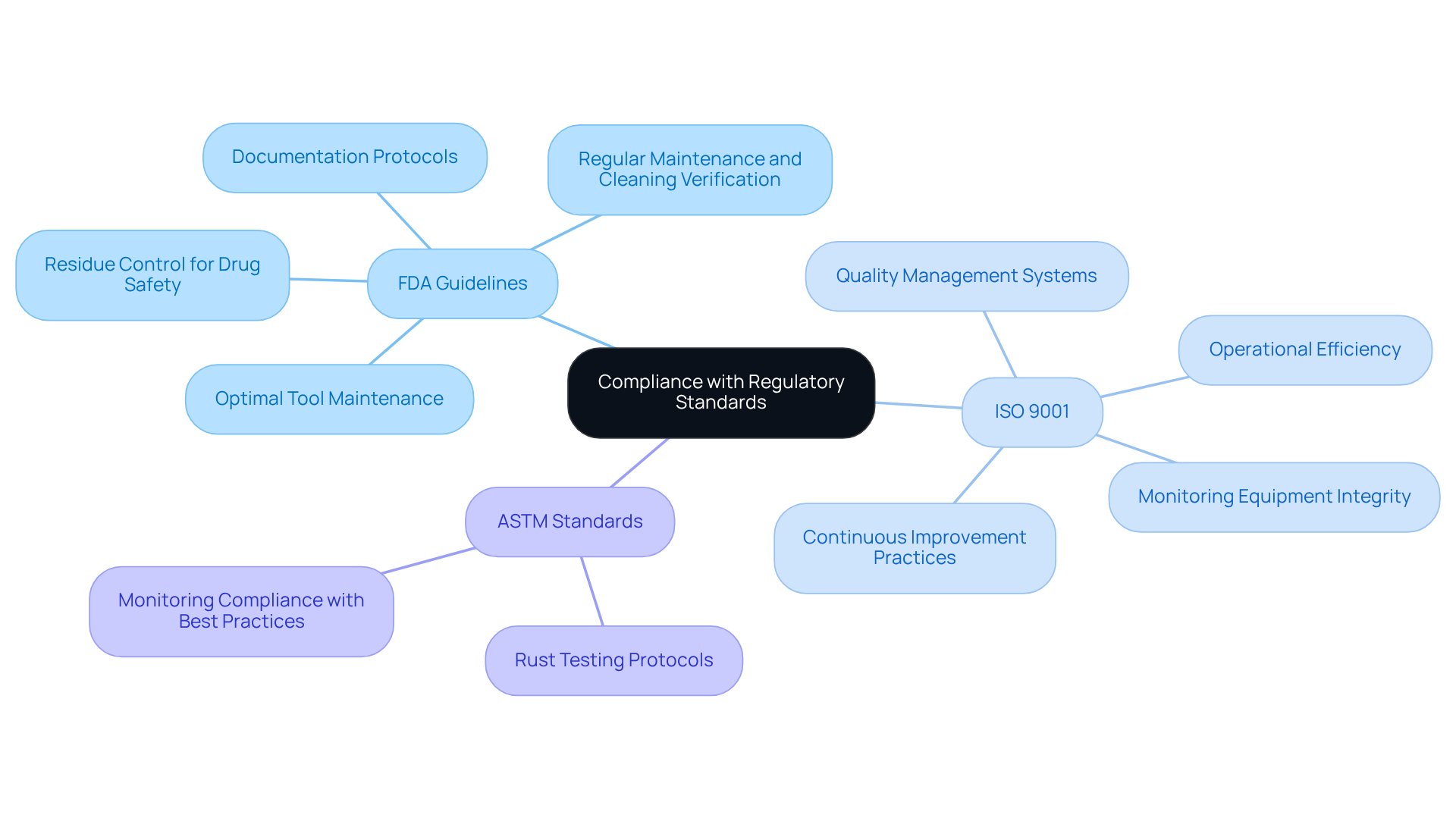
Pharmaceutical laboratories are required to adhere to stringent regulatory standards regarding corrosion measurement as well as machinery maintenance. Key standards that must be observed include:
- FDA Guidelines: The FDA mandates that all tools utilized in drug manufacturing be maintained in optimal condition to avert contamination. This includes regular maintenance, verification of cleaning methods, and ensuring that all GMP-relevant tools are qualified before use, supported by documented protocols and reports. Such diligence is crucial to guarantee that no residues adversely affect drug safety or efficacy.
- ISO 9001: This standard underscores the importance of quality management systems, compelling facilities to establish processes for monitoring and maintaining equipment integrity. Compliance with ISO 9001 not only enhances operational efficiency but also fosters continuous improvement in quality practices.
- ASTM Standards: ASTM International provides comprehensive protocols for rust testing and monitoring, which facilities should adhere to in order to ensure compliance with industry best practices.
Regular audits and meticulous documentation of corrosion measurement practices are vital for demonstrating compliance and sustaining accreditation. These practices not only fulfill regulatory requirements but also bolster the overall integrity and reliability of operational processes. Furthermore, it is imperative to recognize common pitfalls in machinery maintenance, such as insufficient documentation or neglecting routine calibration and preventive upkeep, as these can precipitate compliance issues.
Train Staff on Best Practices and Technologies
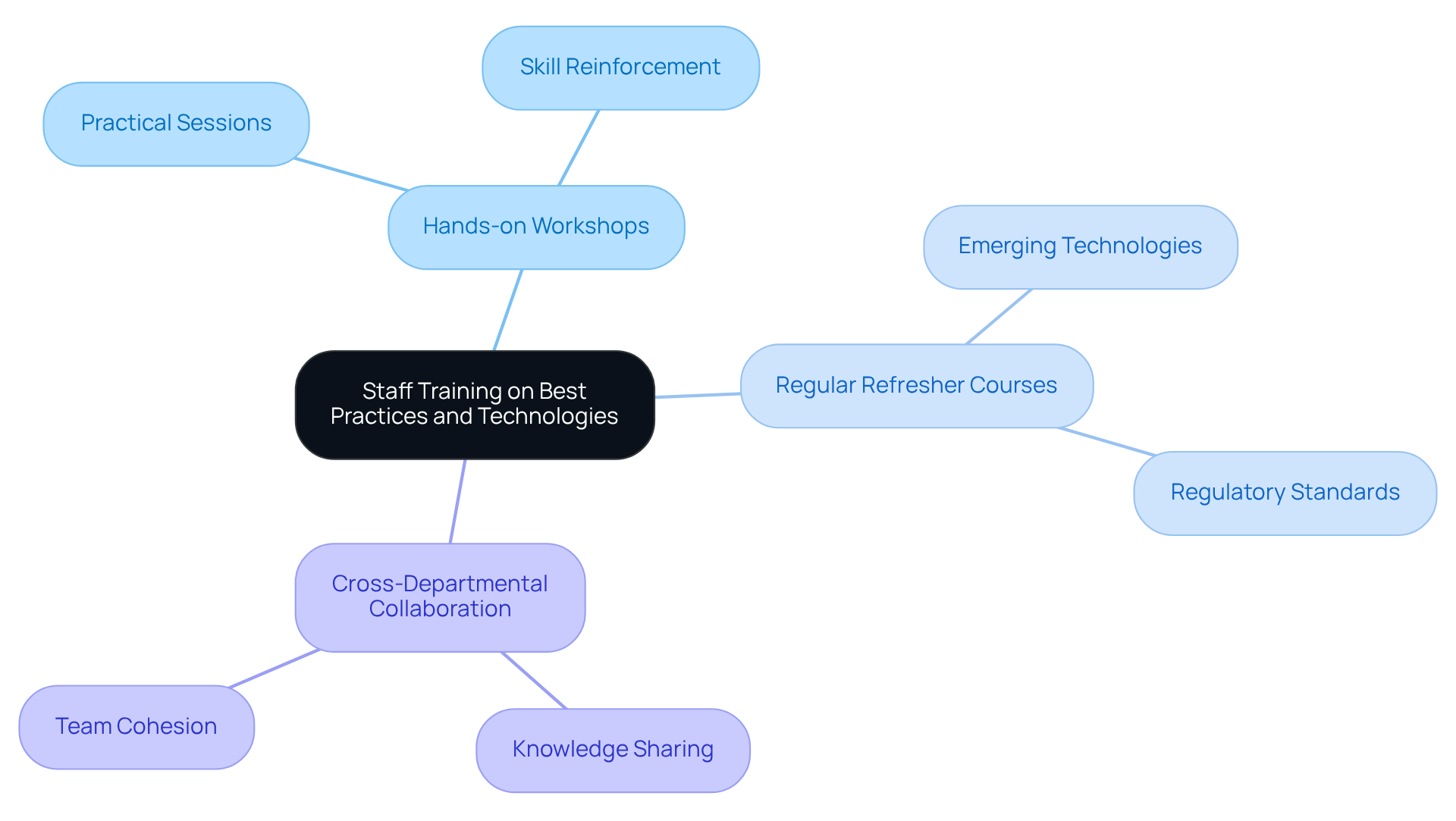
To enhance deterioration assessment methods, it is crucial to equip laboratory personnel with the latest best practices and technologies through focused training programs. An effective training program comprises several key components:
-
Hands-on Workshops: Implementing practical sessions enables staff to engage directly with advanced measurement equipment, fostering skills in accurate operation and result interpretation. This experiential learning is vital for reinforcing theoretical knowledge and enhancing practical competencies. As noted, "training improves productivity and job satisfaction, making it a win-win situation for both employees and employers."
-
Regular Refresher Courses: Ongoing education is essential to keep staff informed about emerging technologies and evolving regulatory standards. Continuous learning sharpens skills and promotes a culture of accountability and excellence within the laboratory environment. Strong documentation of cross-training signoff is critical for assessing competency.
-
Cross-Departmental Collaboration: Facilitating knowledge sharing across departments disseminates best practices related to corrosion measurement. This collaborative approach enhances team cohesion and ensures that all staff members are aligned with the laboratory's operational goals. The "Cross-Training Enhancements in Laboratory Operations" case study illustrates how cross-training can lead to improved operations and a better understanding among staff.
Investing in comprehensive staff training significantly boosts laboratory efficiency and upholds rigorous quality control standards, ultimately leading to improved outcomes in pharmaceutical research and development.
Conclusion
Understanding and managing corrosion in pharmaceutical laboratories is not merely a technical necessity; it is a fundamental aspect of ensuring product safety and operational integrity. This article underscores the critical importance of corrosion measurement as a means to protect valuable laboratory equipment, maintain compliance with stringent regulatory standards, and ultimately safeguard public health. By recognizing the various types of corrosion and their implications, laboratories can implement effective measurement strategies that mitigate risks and enhance the longevity of their assets.
Key techniques such as:
- Electrochemical Impedance Spectroscopy
- Linear Polarization Resistance
- Weight Loss Coupons
- Ultrasonic Thickness Measurement
are vital tools for real-time monitoring and assessment. Each method presents unique advantages, from non-destructive testing to rapid evaluations, empowering laboratories to make informed decisions regarding maintenance and interventions. Furthermore, adherence to regulatory standards like FDA guidelines and ISO 9001 ensures that these practices not only meet compliance requirements but also foster a culture of continuous improvement and quality assurance.
In light of these insights, it is imperative for pharmaceutical labs to prioritize corrosion measurement as an integral part of their operational strategy. Investing in advanced measurement technologies and comprehensive staff training will enhance the effectiveness of corrosion management while contributing to the overall safety and reliability of pharmaceutical products. By taking proactive steps in corrosion measurement, laboratories can protect their investments and uphold the highest standards of quality in their research and development efforts.




