Overview
Detecting the presence of an ion in a solution is crucial for various applications, and it can be accomplished through several methods. These include:
- Conductivity measurements
- Chemical reactions
- Advanced techniques such as ion chromatography
- Mass spectrometry
Each method leverages the unique properties of ions, including their charge and concentration, to provide reliable identification and quantification. This capability is essential in fields like environmental monitoring and pharmaceuticals, where precision is paramount. Understanding these detection methods not only highlights their significance but also underscores the importance of high-quality scientific instruments in laboratory settings.
Introduction
Understanding the presence of ions in a solution is crucial across various scientific fields, including environmental monitoring and pharmaceutical analysis. Accurately detecting these charged particles can unlock valuable insights into chemical compositions and reactions.
Yet, with numerous detection methods available, how does one identify the most effective approach for specific ions? This article explores the principles and techniques of ion detection, offering a comprehensive step-by-step guide that not only highlights opportunities for accurate analysis but also addresses common challenges encountered in the process.
Understand Ion Detection Principles
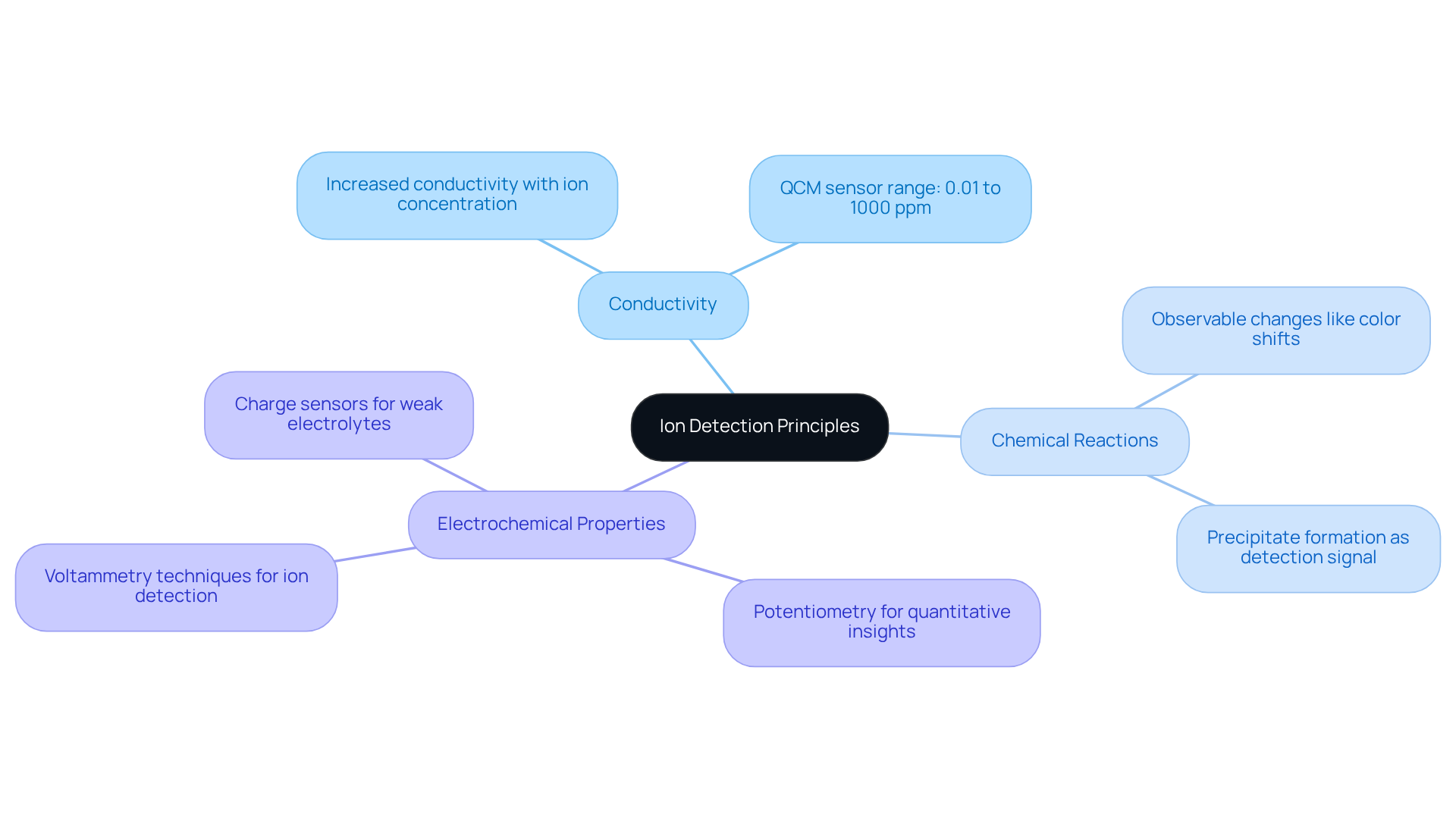
The identification of charged particles is fundamentally reliant on their behavior within a solution, prompting inquiries into how can the presence of an ion in a solution be detected, influenced by factors such as concentration, charge, and the presence of additional substances. Ions, categorized as cations (positively charged) or anions (negatively charged), engage with various reagents, making the comprehension of these interactions essential for effective detection. Key principles to consider include:
- Conductivity: The conductivity of a solution directly correlates with the concentration of ions. Elevated levels of ions lead to increased conductivity, making it a critical factor in assessing ion presence. For instance, the range of trace metal ion adsorption by a QCM sensor spans from 0.01 to 1000 ppm, highlighting the importance of ion concentrations in conductivity measurements.
- Chemical Reactions: Many identification techniques leverage specific chemical reactions that yield observable changes, such as color shifts or precipitate formation, which signal the presence of particular elements.
- Electrochemical Properties: Techniques such as potentiometry and voltammetry evaluate the electrical properties of ions in solution, providing quantitative insights into their concentrations. Notably, charge sensors exhibit a more pronounced response for weak electrolytes compared to conventional conductivity sensors, thereby enhancing the accuracy of ion identification.
Understanding how can the presence of an ion in a solution be detected is vital for selecting the appropriate identification method and accurately interpreting results. Recent studies underscore the necessity of grasping ion behavior in solutions to advance identification technologies and ensure reliable measurements across various applications, from environmental monitoring to pharmaceutical analysis. The ongoing global water crisis accentuates the urgent need for effective detection techniques for heavy metals, rendering this understanding even more pertinent.
Explore Methods for Detecting Cations and Anions
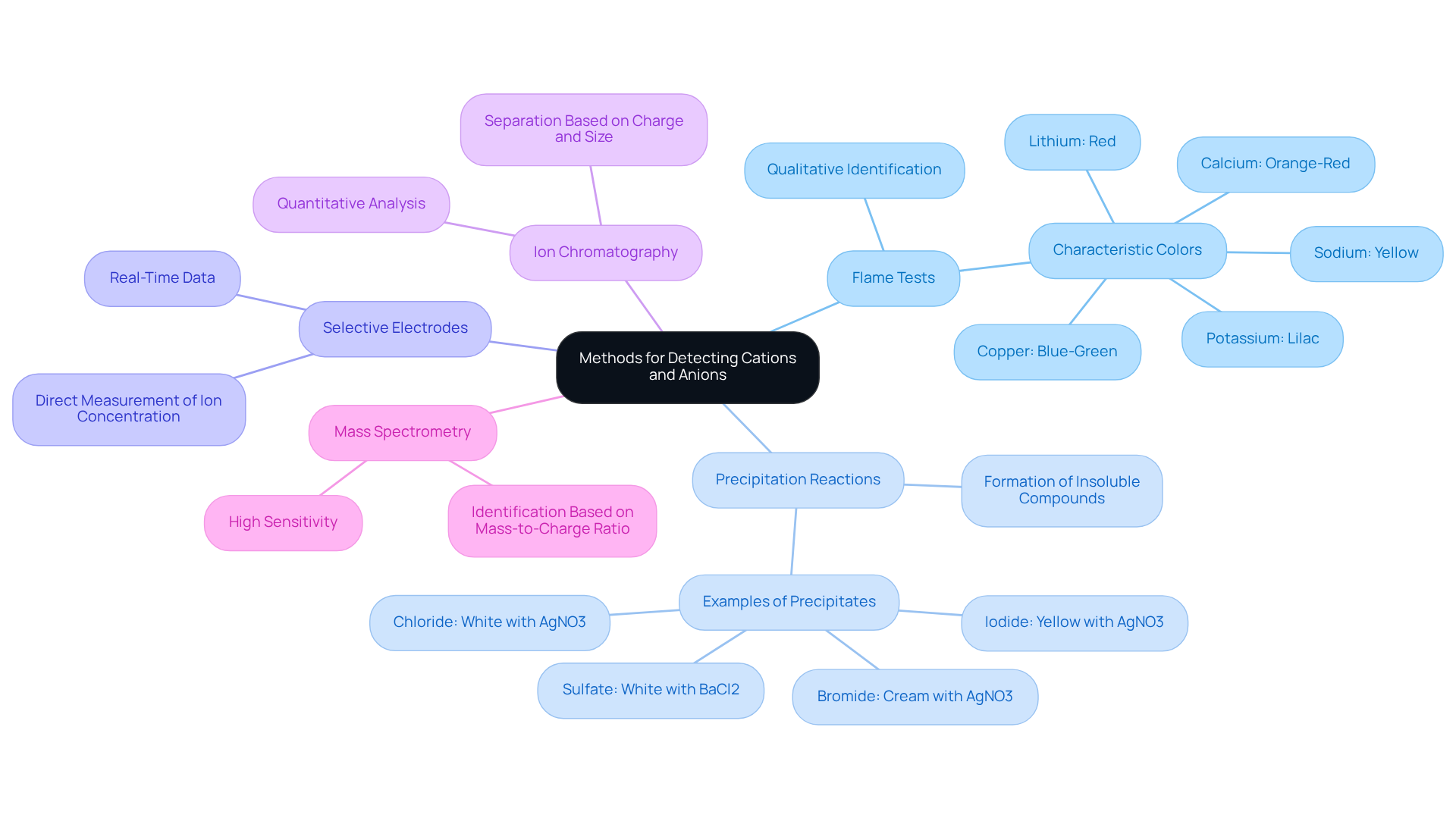
In various scientific fields, identifying cations and anions is essential, and it raises the question of how can the presence of an ion in a solution be detected, with several techniques available, each having distinct advantages and limitations. Understanding how can the presence of an ion in a solution be detected is crucial for effective analysis and application in laboratory settings.
-
Flame Tests serve as a qualitative approach, where introducing a sample into a flame reveals a characteristic color. Different metal ions produce specific hues, facilitating their identification. This method is straightforward yet effective for preliminary assessments on how can the presence of an ion in a solution be detected.
-
Precipitation Reactions involve adding targeted reagents to a solution, leading to the formation of insoluble compounds or precipitates. These can be filtered and analyzed, which raises the question of how can the presence of an ion in a solution be detected. This technique is particularly useful for isolating specific ions from complex mixtures, which raises the question of how can the presence of an ion in a solution be detected.
-
Selective Electrodes offer a direct measurement of ion concentration by responding specifically to certain particles in a solution. This method enhances accuracy and provides real-time data, which is crucial for understanding how can the presence of an ion in a solution be detected in precise analytical work.
-
Ion Chromatography is a sophisticated technique that separates charged particles based on their charge and size. This allows for quantitative analysis of both cations and anions within a mixture, which helps answer how can the presence of an ion in a solution be detected, making it a preferred choice for detailed investigations.
-
Mass Spectrometry stands out as a highly sensitive method capable of identifying and quantifying particles based on their mass-to-charge ratio. This technique is particularly suited for analyzing complex mixtures, offering unparalleled precision.
Each of these techniques finds application in different scenarios, and understanding how can the presence of an ion in a solution be detected depends on factors such as the specific particles in question, the required sensitivity, and the available laboratory equipment. Understanding these options empowers scientists to select the most appropriate approach for their analytical needs.
Follow Step-by-Step Procedures for Ion Detection
To effectively detect ions in a solution, it is essential to adhere to a structured approach:
-
Preparation of Samples: Begin by dissolving the unknown sample in deionized water to create a solution, ensuring that its concentration aligns with the selected detection technique.
-
Choice of Detection Technique: Select an appropriate method based on the anticipated cations or anions and the available analytical instruments. In pharmaceutical laboratories, techniques such as ion-selective electrodes or ion chromatography are frequently utilized to illustrate how can the presence of an ion in a solution be detected due to their precision and reliability.
-
Conducting the Test:
- For Flame Tests: Introduce a small sample into a flame and observe the resulting color, comparing it against known standards for accurate identification.
- For Precipitation Reactions: Add a reagent that reacts with the target ion to form a precipitate. Filter the solution to collect this precipitate for further analysis.
- For Ion-Selective Electrodes: Calibrate the electrode according to the manufacturer's guidelines, immerse it in the solution, and record the potential to determine ion concentration accurately.
- For Ion Chromatography: Inject the prepared sample into the chromatograph, analyzing the output based on the retention times of the ions, which can vary significantly depending on the technique employed.
-
Data Analysis: Interpret the results according to the method utilized. For quantitative analyses, compare findings to calibration standards to determine how can the presence of an ion in a solution be detected with precision.
-
Documentation: Meticulously record all observations, results, and any deviations from expected outcomes to ensure compliance with laboratory protocols and facilitate future reference.
Incorporating best practices in sample preparation is crucial for achieving reliable results. Ensuring that samples are free from contaminants can significantly enhance the accuracy of identification. Real-world examples from pharmaceutical laboratories underscore the importance of selecting the right method based on the specific ions being analyzed, as this choice can greatly impact both the efficiency and accuracy of the results. By diligently following these steps, laboratories can enhance their ion identification processes, ultimately leading to improved analytical outcomes.
Troubleshoot Common Issues in Ion Detection
In the realm of ion identification, several common challenges can impede accurate results. Addressing these issues is critical for maintaining the integrity of scientific processes.
-
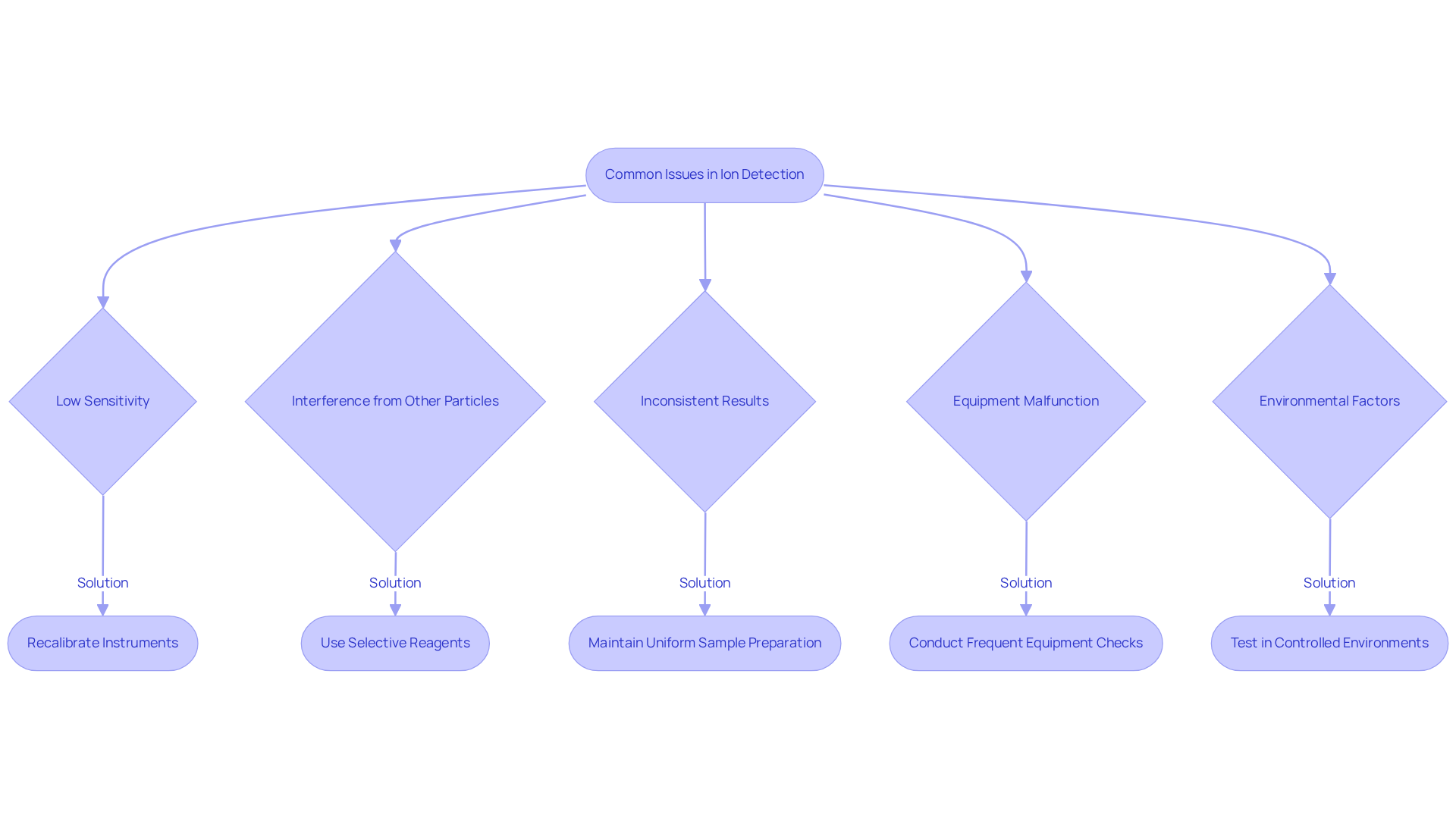
Low sensitivity can significantly hinder detection methods, yielding subpar results. To counteract this, recalibrating instruments is essential, alongside verifying that reagents are fresh and correctly prepared. Routine calibration is not merely a best practice; research indicates that improper calibration can greatly impact detection thresholds.
-
Interference from other particles presents another challenge. The presence of specific particles can disrupt the identification of target ions. Employing selective reagents or methodologies specifically designed to differentiate between ions minimizes cross-reactivity and enhances accuracy.
-
Inconsistent results often arise from variability in sample preparation. To ensure reliability, it is imperative that sample handling remains uniform and that all equipment is maintained according to strict protocols. Regular control sample runs can help identify when recalibration is necessary, thus enhancing the reliability of results.
-
Equipment malfunction is a prevalent issue that can compromise outcomes. Frequent checks and maintenance of equipment, including electrodes and chromatographs, are vital. Industry statistics reveal significant rates of equipment malfunctions in ion sensing processes, underscoring the necessity for a rigorous maintenance schedule. As noted by experts at Inorganic Ventures, "Regular maintenance and calibration are essential to ensure accurate and reliable measurements."
-
Environmental factors such as temperature and humidity can also affect ion behavior and measurement accuracy. Conducting tests in controlled environments is crucial to mitigate these effects.
By adhering to these troubleshooting strategies, laboratories can significantly enhance the reliability of their ion detection processes, ensuring accurate and consistent results.
Conclusion
Understanding the presence of ions in a solution is crucial for a multitude of scientific applications, ranging from environmental monitoring to pharmaceutical analysis. This article delves into the principles of ion detection, emphasizing the importance of factors such as conductivity, chemical reactions, and electrochemical properties. By exploring diverse methods for identifying both cations and anions, it highlights the significance of selecting the appropriate technique based on specific requirements and available resources.
Key techniques discussed include:
- Flame tests
- Precipitation reactions
- Selective electrodes
- Ion chromatography
- Mass spectrometry
Each technique offers unique advantages for ion detection. Furthermore, the article outlines a structured approach to ion detection, covering:
- Sample preparation
- Method selection
- Testing procedures
- Data analysis
- Documentation
Troubleshooting common issues ensures that laboratories can maintain the integrity of their results, addressing challenges such as low sensitivity, interference from other particles, and environmental factors.
Ultimately, the ability to accurately detect ions in solutions transcends mere technical skill; it is a vital necessity for advancing scientific research and ensuring safety across various industries. By mastering these detection methods and troubleshooting strategies, scientists can enhance their analytical capabilities, contributing to more reliable outcomes in their work. The ongoing demand for precision in ion detection underscores the importance of continual learning and adaptation within this field.




