Overview
Titration is a critical technique in analytical chemistry, characterized by the incremental addition of a solution with a known concentration, known as the titrant, to an unknown solution, referred to as the analyte. This process continues until the chemical reaction reaches its endpoint, which allows for the precise determination of the analyte's concentration. The article delves into essential principles of titration, various methods employed, and detailed step-by-step procedures. It underscores the paramount importance of accuracy and precision in measurements, particularly within pharmaceutical applications. Such diligence ensures reliable experimental outcomes, reinforcing the necessity for high-quality scientific instruments in laboratory settings.
Introduction
In the realm of analytical chemistry, titration emerges as a cornerstone technique, indispensable for accurately determining the concentration of unknown solutions. This method serves not only as a fundamental tool across various scientific disciplines—including biology and environmental science—but also plays a pivotal role in maintaining the integrity of research and quality control in laboratories. As titration techniques advance alongside technological innovations, such as automated systems designed for pharmaceutical applications, the importance of this method is amplified. By comprehending the principles and types of titration, and mastering the procedural intricacies, researchers are equipped with the essential skills to navigate the complexities of quantitative analysis. This ultimately leads to reliable and reproducible results, reinforcing the critical nature of titration in scientific inquiry.
Define Titration: Principles and Importance
To understand how titration works, it is important to recognize that it is a fundamental quantitative analytical technique used to determine the concentration of an unknown solution (analyte) through the incremental addition of a solution of known concentration (titrant) until the reaction reaches its endpoint. This method is pivotal across various scientific disciplines, including chemistry, biology, and environmental science, as it enables precise measurements of chemical concentrations. The importance of this analytical technique is underscored by its capacity to yield precise and consistent outcomes, essential for maintaining research integrity and quality assurance in laboratory settings.
Recent advancements in measurement techniques have significantly enhanced their applicability, particularly within pharmaceutical research, where precision is paramount. For instance, the integration of automated dosing systems, such as the AQ-300 Coulometric Karl Fischer apparatus and the Hiranuma Aquacounter AQV-300 Volumetric device, has optimized processes, minimizing human error while enhancing throughput. These specialized devices are meticulously designed to comply with the stringent requirements of drug and medicine testing as outlined in the Japanese Pharmacopoeia, ensuring that pharmaceutical products adhere to the highest standards of quality and safety.
Understanding how titration works is essential for researchers aiming to ensure the reliability of their experimental data in volumetric analysis. Key principles include:
- Understanding the stoichiometry of the reaction
- The selection of appropriate indicators
- The meticulous calibration of instruments
Collectively, these components bolster the precision of measurements, which are critical for making informed decisions based on experimental outcomes.
Expert opinions further highlight the significance of this method in analytical chemistry, with many experts deeming it a fundamental technique for quantitative analysis. As Endler Marcel Borges noted, "The results are compared with subsequent simultaneous potentiometric and colorimetric analyses using EDTA," thereby validating the reliability of these techniques. Recent studies emphasize that the continuous advancement of measurement techniques and devices, including those provided by JM Science Inc., is vital for meeting the evolving demands of laboratories, particularly within the pharmaceutical industry, where precision and accuracy are indispensable. The depth of research supporting the importance of this analytical method is also evident, with references to 60 other publications enriching the body of knowledge in chemical education.
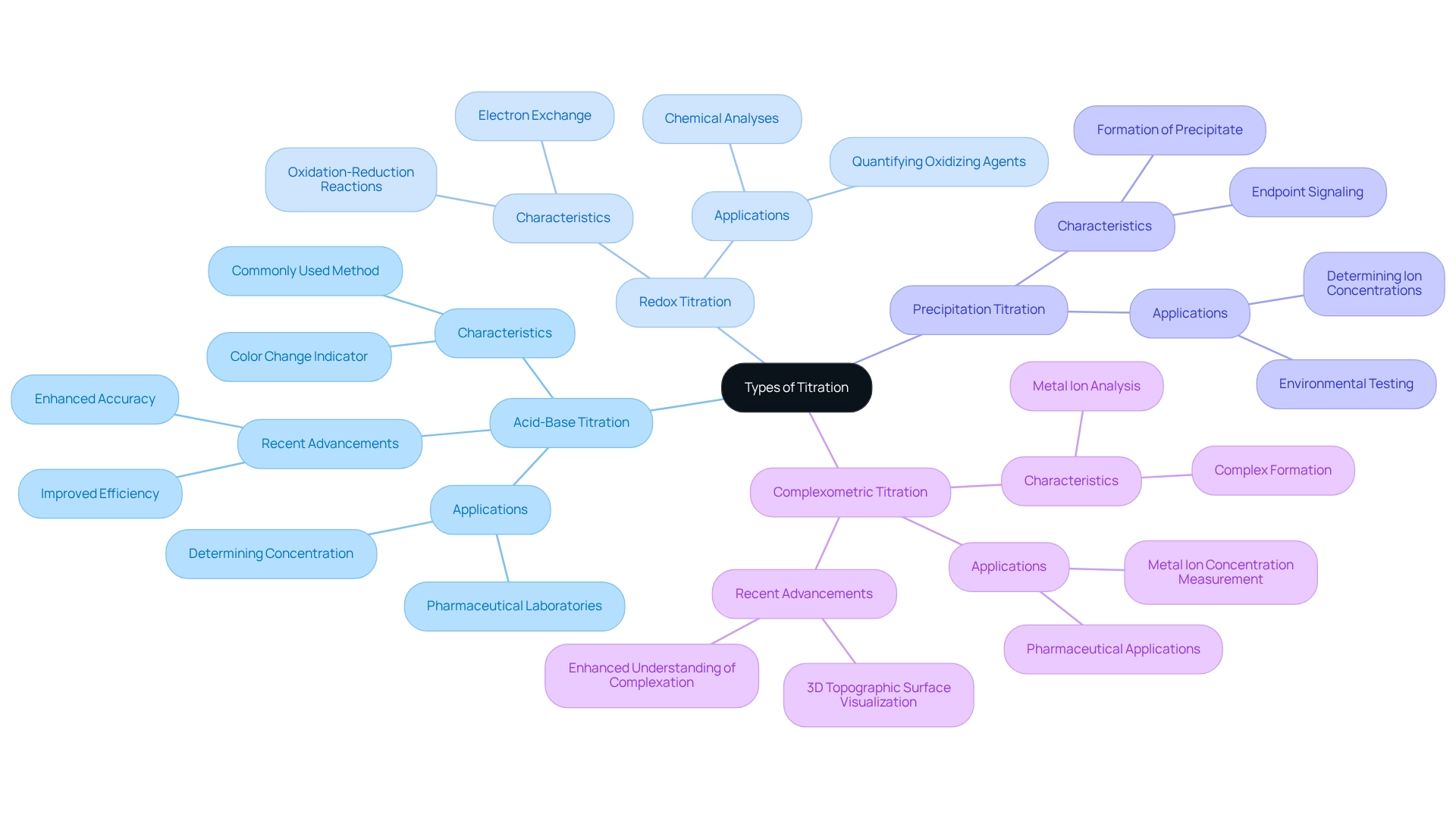
Explore Types of Titration: Acid-Base, Redox, and More
A fundamental analytical technique, titration raises the question of how does titration work, encompassing several types specifically tailored to distinct chemical reactions and applications.
Acid-Base Titration is the most prevalent method, wherein an acid engages in a reaction with a base. The endpoint is typically indicated by a color change from a pH indicator, allowing chemists to determine the concentration of an unknown solution with ease. Recent advancements in acid-base titration techniques have significantly enhanced accuracy and efficiency, particularly within pharmaceutical laboratories where precise measurements are critical. The commitment to understanding and mitigating errors reinforces the reliability of individual experiments, a cornerstone in this field.
Redox Titration involves oxidation-reduction reactions, characterized by the exchange of electrons between the reagent and analyte. This method proves particularly useful for quantifying the concentration of oxidizing or reducing agents, a necessity in various chemical analyses.
Precipitation Titration employs a method where a precipitate forms during the reaction, signaling the endpoint. This approach is invaluable for determining ion concentrations in solution, especially within environmental and quality control testing.
Complexometric Titration relies on the formation of a complex between the titrant and the analyte, commonly utilized for metal ion analysis. This technique holds particular relevance in pharmaceutical applications, where accurate measurement of metal ion concentrations is imperative. The visualization of metal ion buffering in complexometric assessments has been enhanced through software that generates 3D topographic surfaces, aiding in the understanding of complexation phenomena. This innovative approach exemplifies the ongoing evolution in measurement techniques, ensuring laboratories achieve precise and reliable results.
Understanding how does titration work enables chemists to choose the most suitable technique based on the chemical characteristics of the substances involved. As Robert H. Grubbs, a noted chemist, articulated, "The key to scientific discovery is the ability to recognize and address errors." This underscores the critical importance of error management in measurement processes, reinforcing the reliability of experimental results.
Conduct a Titration: Step-by-Step Procedure
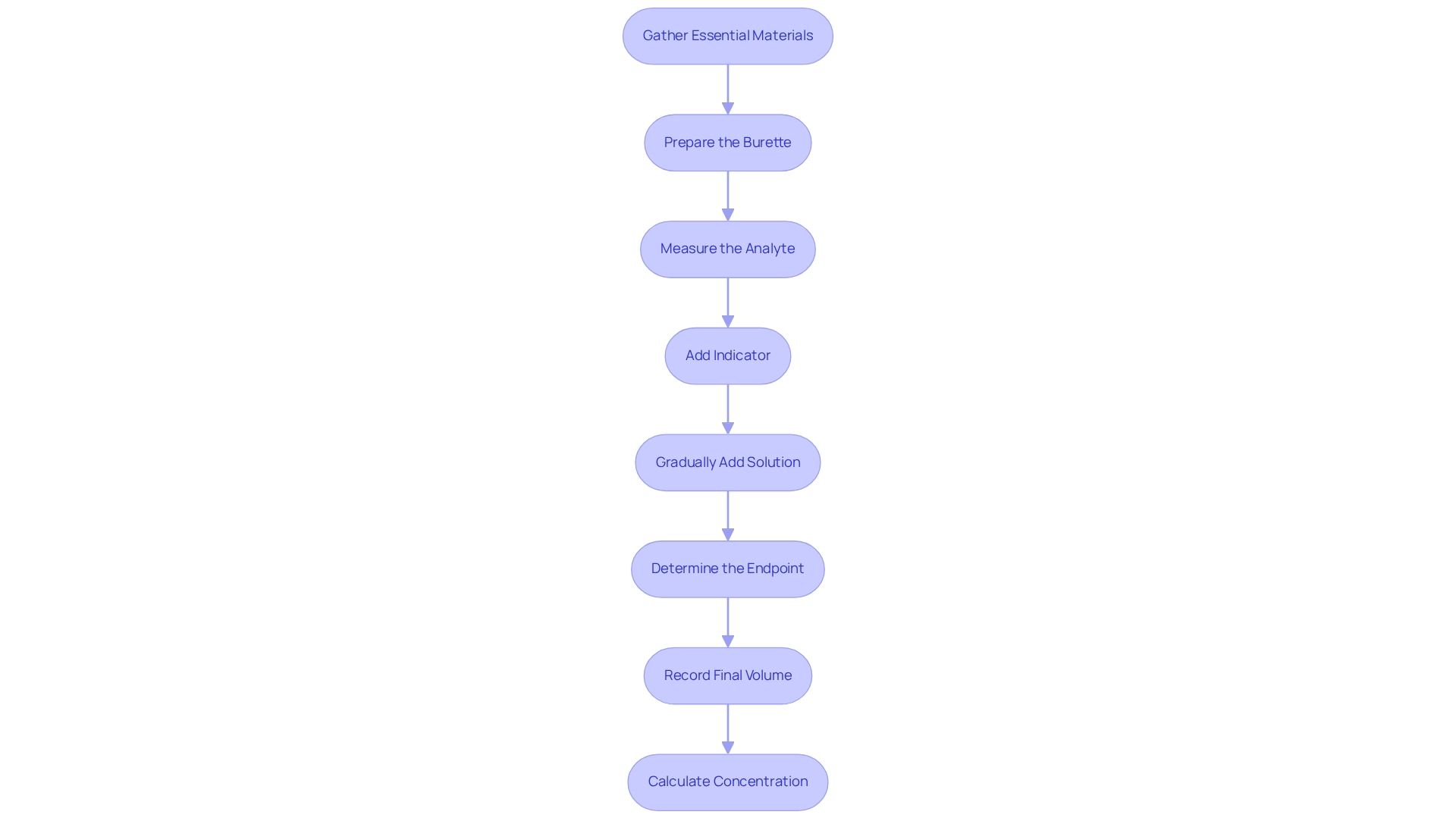
To conduct a titration effectively, it is essential to adhere to the following step-by-step procedure:
- Gather Essential Materials: Ensure you have a burette, pipette, Erlenmeyer flask, reagent, analyte, and an appropriate indicator.
- Prepare the burette: Rinse the burette with the reagent liquid to avoid contamination. Fill the burette with the titrant and accurately record the initial volume.
- Measure the Analyte: Utilize a pipette to measure a precise volume of the analyte liquid, transferring it to the Erlenmeyer flask.
- Add Indicator: Introduce a few drops of the visual indicator to the analyte solution in the flask, if applicable.
- Gradually add the solution from the burette to the analyte while continuously swirling the flask. Monitor for a color change or other endpoint indicators.
- Determine the Endpoint: Cease adding the reagent when the endpoint is reached, indicated by a stable color change or another signal.
- Record Final Volume: Document the final volume of the solution in the burette and calculate the volume used.
- Calculate Concentration: Use the formula for neutralization to determine the concentration of the analyte based on the volume of reagent used and its concentration.
By carefully adhering to these steps, one can obtain precise and dependable results, which are crucial for effective statistical analysis in drug development and for understanding how does titration work in other scientific applications. As William V. Rossi states, "Statistical analysis is at the heart of drug discovery and development, guiding decisions that can impact patient outcomes."
Laboratory supervisors emphasize that accuracy in measurement not only improves data integrity but also aids informed decision-making in research and clinical trials. Furthermore, integrating best practices for performing analyses in 2025 can enhance precision and effectiveness in laboratory environments. Real-world instances of successful measurements demonstrate the practical application of these methods, underscoring their significance in obtaining reliable outcomes.
Troubleshoot Titration: Common Issues and Solutions
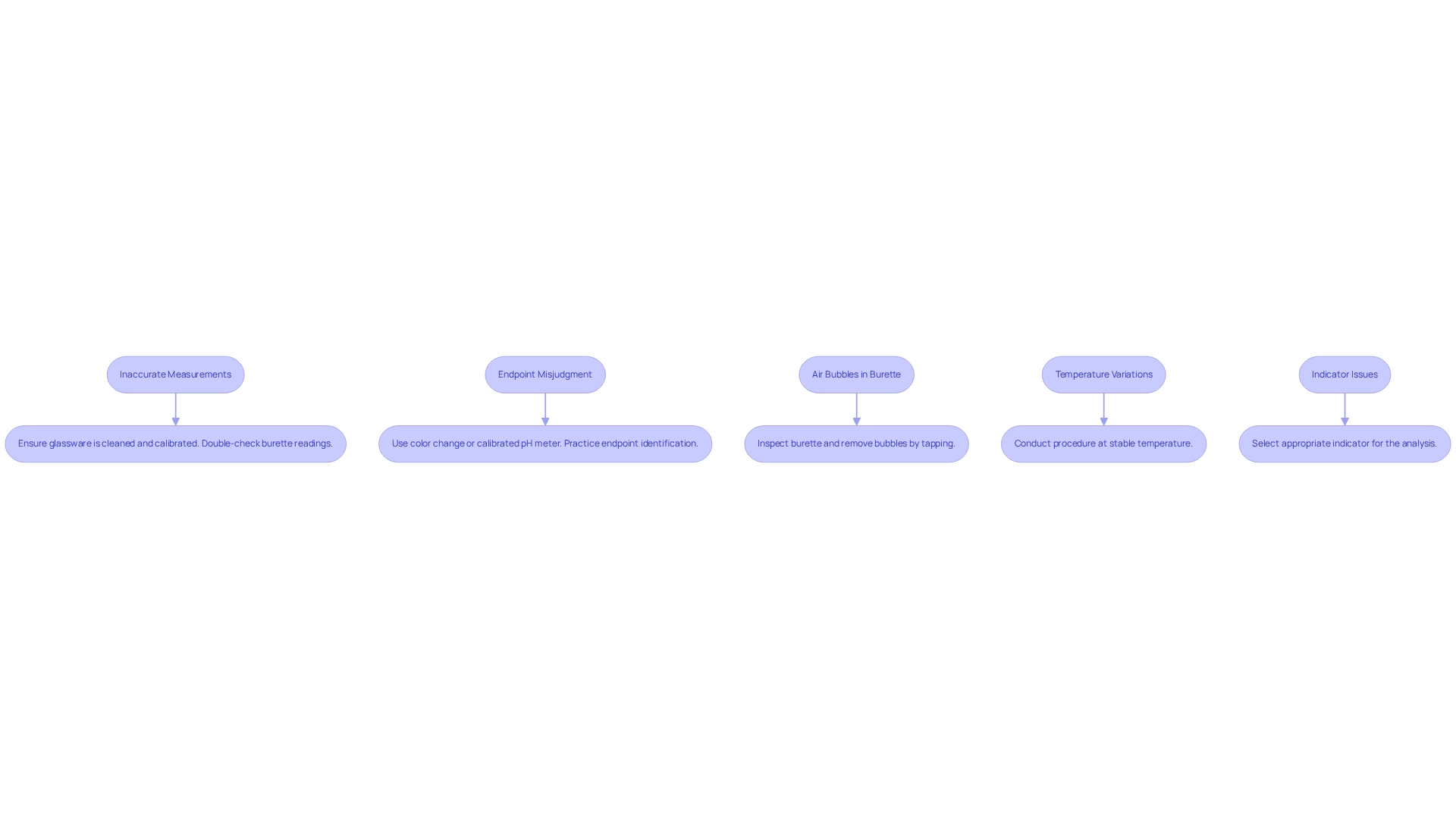
Frequent challenges encountered during laboratory processes can significantly impact the precision and reliability of findings. Addressing these obstacles is crucial for achieving accurate measurements in pharmaceutical labs. Below are some common issues and their corresponding solutions:
- Inaccurate Measurements: It is imperative to ensure that all glassware is meticulously cleaned and calibrated. Misreading the burette can lead to substantial errors; therefore, double-checking readings is essential.
- Endpoint Misjudgment: Consistency in determining the endpoint is vital. Employing methods such as a color change or a calibrated pH meter can enhance accuracy. Regular practice in identifying the endpoint further improves outcomes.
- Air Bubbles in Burette: Air bubbles trapped in the burette tip can distort the volume delivered. Always inspect the burette prior to beginning the process and remove any bubbles by gently tapping the side.
- Temperature Variations: Conduct the procedure at a stable temperature, as fluctuations can alter reaction rates and solubility, leading to unreliable results. Research indicates that temperature variations can significantly affect measurement precision, underscoring the importance of maintaining a controlled environment.
- Indicator Issues: Selecting the appropriate indicator for the specific analysis is essential. Certain indicators may not yield a clear endpoint for specific reactions, thus necessitating a solid understanding of the underlying chemistry.
By recognizing these prevalent challenges and implementing the suggested measures, laboratories can significantly enhance the reliability of their analytical results. Additionally, a thorough review of repeated trial findings is recommended to address discrepancies and refine techniques. As highlighted in the case study 'Procedural and Technique Errors in Acid-Base Titrations,' understanding how does titration work, including practices like pre-titration preparation, can enhance procedural integrity and improve accuracy and reliability. This commitment to precision not only enhances experimental outcomes but also furthers the advancement of scientific knowledge in the field. Remember, "If you don’t make a good solution, you won’t get a good result," emphasizing the critical role of preparation in achieving accurate titration results.
Conclusion
In conclusion, mastering titration techniques is not merely beneficial but essential for anyone engaged in quantitative analysis. This fundamental method in analytical chemistry is pivotal for accurately determining the concentration of unknown solutions, impacting various scientific disciplines from chemistry to environmental science.
As the field evolves with technological advancements, the foundational principles and procedural integrity of titration remain critical for achieving trustworthy results. Embracing these methods ensures that researchers can confidently navigate the complexities of analytical chemistry, ultimately leading to significant contributions in their respective fields.
The commitment to precision and quality control in laboratory settings underscores the importance of thorough training and practice, reinforcing the role of titration in supporting informed decision-making in research and clinical trials.




