Overview
This article serves as an essential guide for laboratories aiming to implement the NIR equation effectively within their workflows. It outlines critical steps, including:
- The gathering of necessary instruments
- The development of calibration frameworks
- Troubleshooting common issues
Each of these components plays a vital role in enhancing the reliability and accuracy of NIR spectroscopy in analytical processes. By following this comprehensive guide, laboratories can significantly improve their operational efficiency and data integrity.
Introduction
Implementing the NIR equation has the potential to revolutionize laboratory practices, yet many labs face significant challenges with the complexities of near-infrared spectroscopy. This guide offers a comprehensive, step-by-step approach to mastering the NIR equation, detailing the essential instruments and procedures that enhance analytical accuracy. As labs embark on this transformative journey, they often encounter obstacles that may impede their progress.
What are the common pitfalls in NIR implementation, and how can they be effectively navigated? Understanding these challenges is crucial for successful integration.
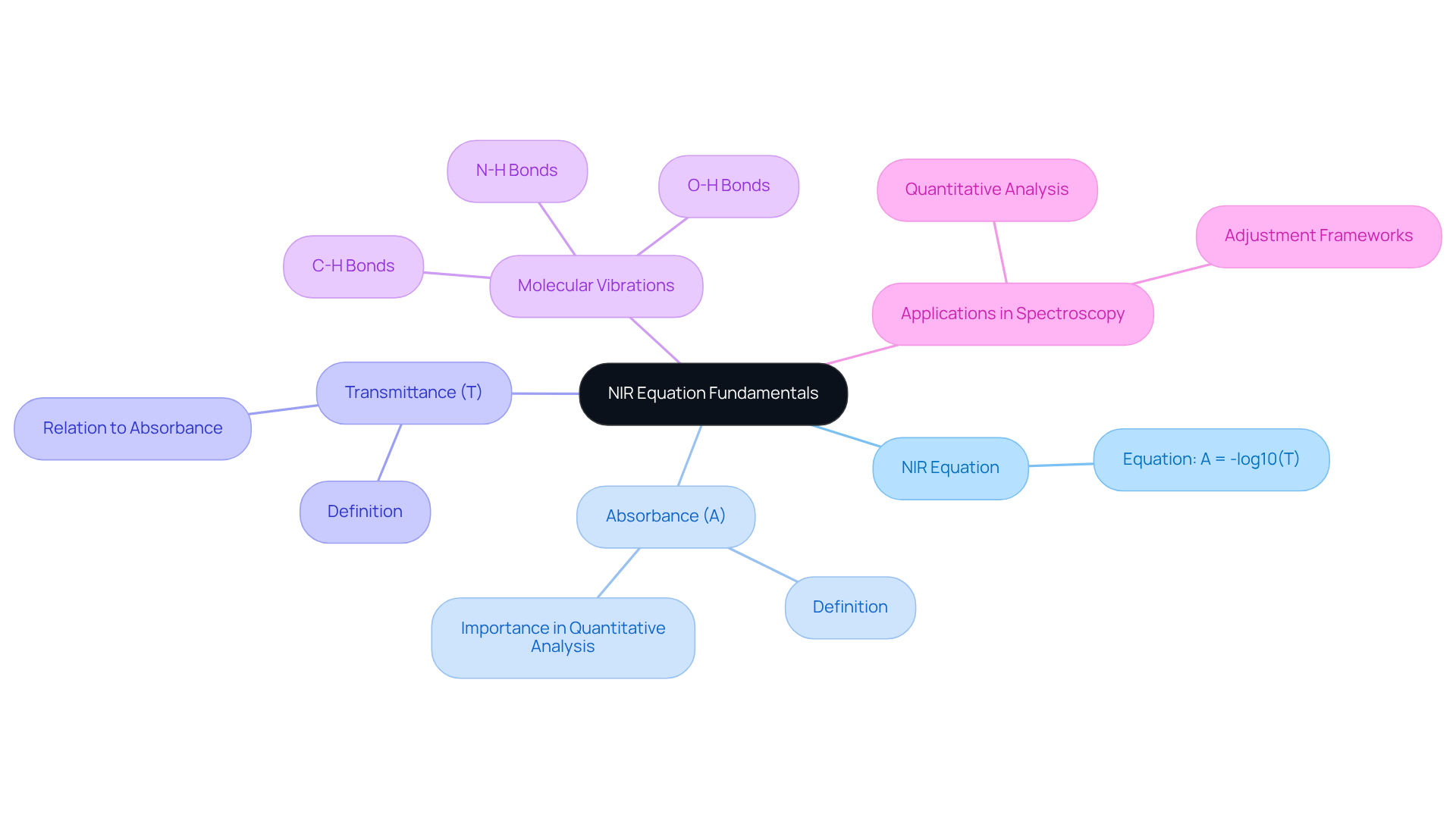
Understand the NIR Equation Fundamentals
The nir equation is based on the absorption of light in the near-infrared spectrum, which usually ranges from 700 nm to 2500 nm. This technique capitalizes on the molecular vibrations of chemical bonds, specifically C-H, O-H, and N-H bonds. The fundamental equation can be expressed as:
A = -log10(T)
Where:
- A represents the absorbance,
- T denotes the transmittance of light through the sample.
Grasping the nir equation is crucial for interpreting the spectra derived from NIR spectroscopy. The absorbance values are directly correlated with the concentration of specific components within the sample, thereby facilitating quantitative analysis. Moreover, familiarity with the nir equation is instrumental in developing adjustment frameworks, which are vital for achieving precise measurements in laboratory processes.
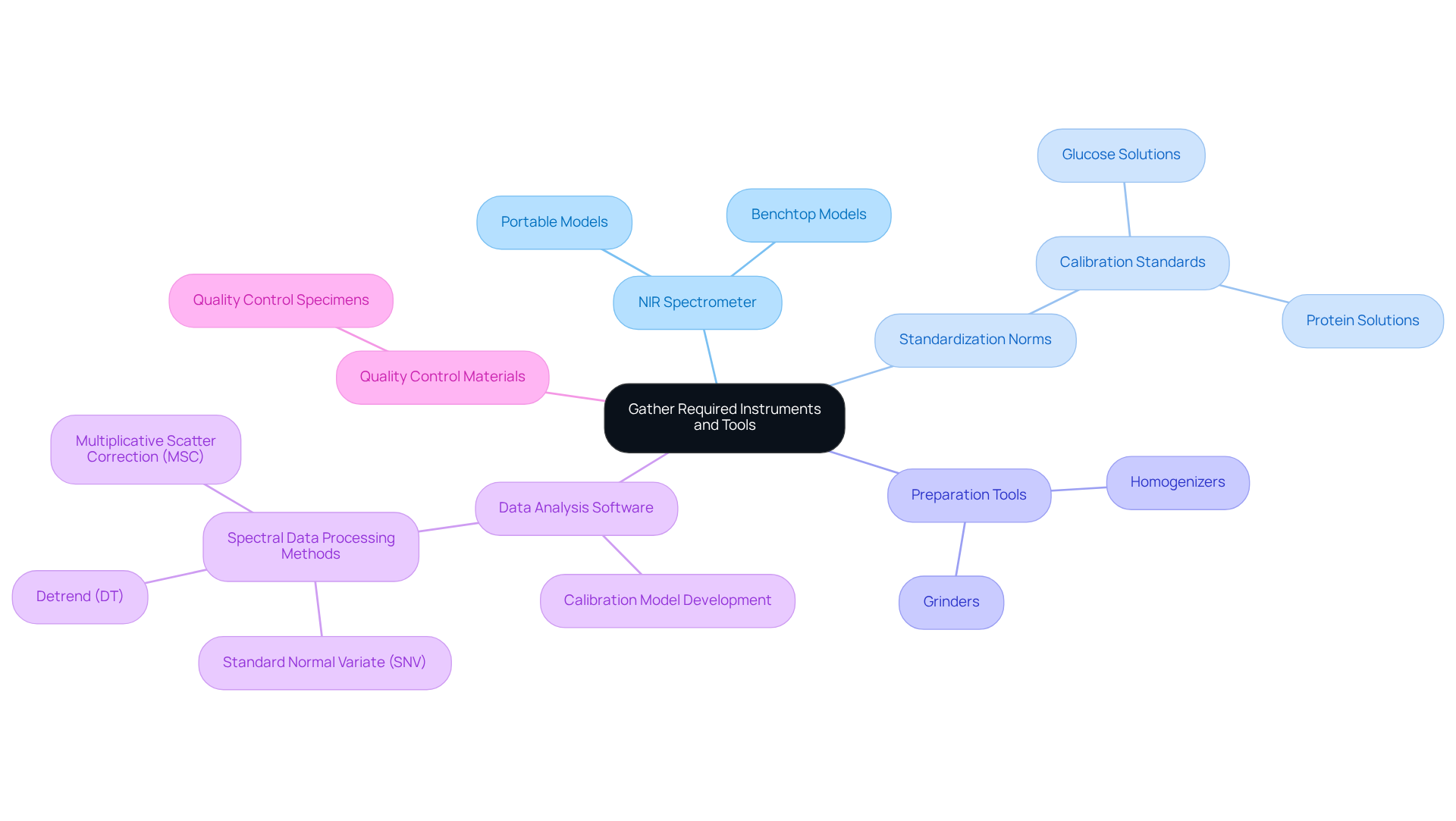
Gather Required Instruments and Tools
To effectively implement the NIR equation in your laboratory, it is essential to gather the following instruments and tools:
-
NIR Spectrometer: This instrument serves as the cornerstone for measuring the absorbance of near-infrared light. Select a model that aligns with your specific application, whether it be a portable or benchtop spectrometer. NIRS technology is recognized for its fast, inexpensive, non-destructive, and reliable analytical capabilities, making it a valuable alternative to traditional wet chemistry methods.
-
Standardization Norms: Acquire samples with known concentrations to create standard curves. These standards are crucial for developing precise predictive frameworks and ensuring trustworthy results. Commonly used calibration standards in NIR spectroscopy include materials like glucose and protein solutions, which contribute to the creation of robust calibration models.
-
Preparation Tools: Depending on the nature of your specimens, you may need grinders, homogenizers, or other equipment to adequately prepare specimens for analysis. Proper specimen preparation is vital for obtaining precise results in NIR analysis.
-
Data Analysis Software: A robust software solution for processing and analyzing NIR data is vital. Choose programs that facilitate the development and validation of calibration models to enhance analytical accuracy. Familiarity with common spectral data processing methods, such as standard normal variate (SNV), detrend (DT), and multiplicative scatter correction (MSC), will further improve your analysis.
-
Quality Control Materials: Regular performance checks of your NIR system using quality control specimens are necessary to maintain consistent and reliable results. This practice ensures that your NIR spectrometer continues to deliver accurate measurements over time.
By gathering these tools, you will establish a solid foundation for integrating the NIR equation into your laboratory workflow. Additionally, considering the average costs of NIR spectrometers, which can vary significantly based on features and capabilities, will aid in budgeting for your laboratory's needs.
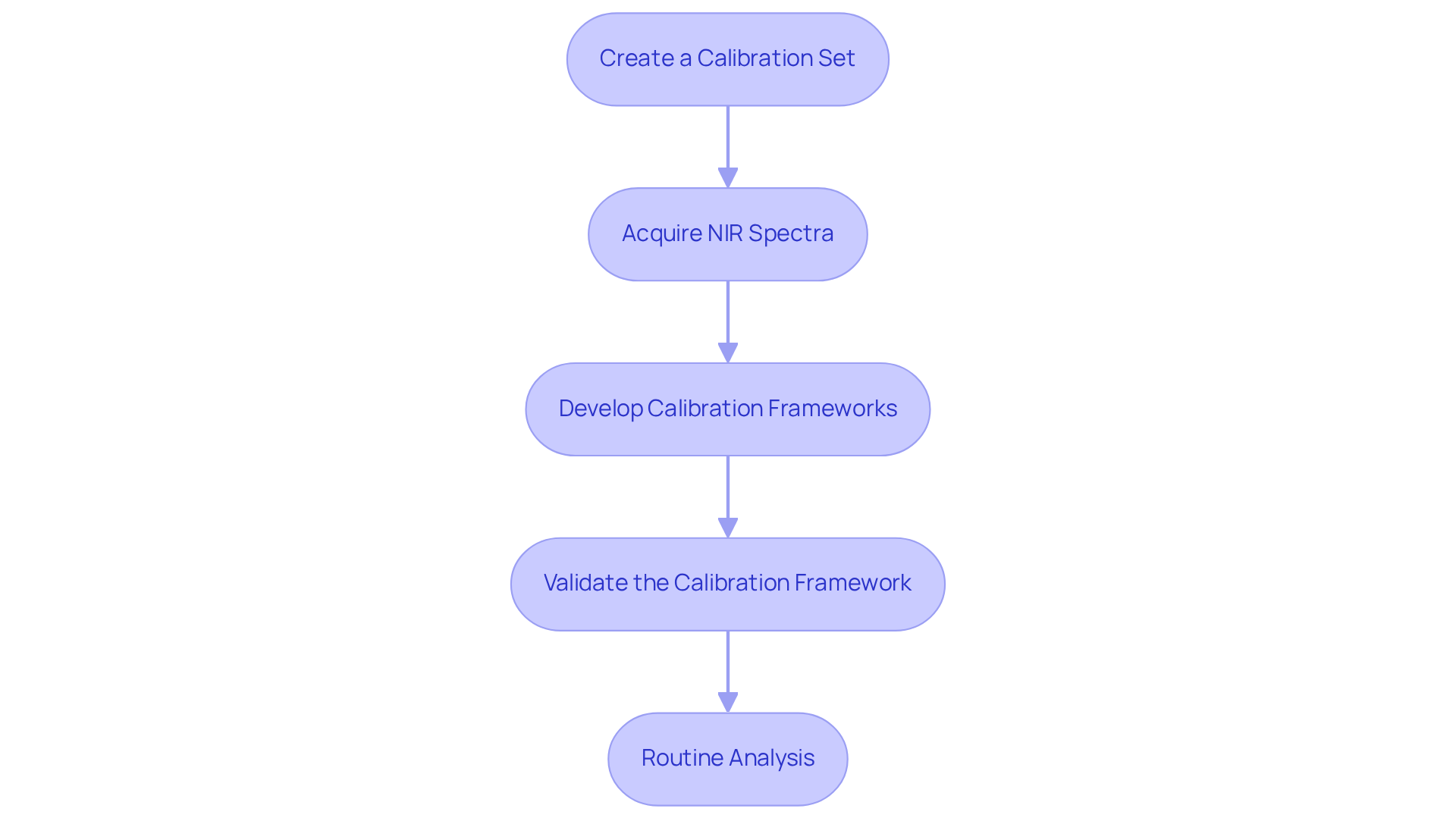
Implement the NIR Equation in Your Workflow
To effectively implement the NIR equation in your laboratory workflow, adhere to the following structured steps:
- Create a Calibration Set: Begin by gathering a diverse range of specimens that contain known concentrations of the analytes of interest. This comprehensive collection will serve as the foundation for your adjustment system.
- Acquire NIR Spectra: Utilize the NIR spectrometer to meticulously measure the absorbance of each sample within your reference set. It is imperative that the instrument is properly calibrated and that environmental conditions are meticulously controlled.
- Develop Calibration Frameworks: Employ data analysis software to construct calibration frameworks that establish a correlation between the NIR spectra and the known concentrations. Common methodologies, such as Partial Least Squares (PLS) regression, are highly effective in this context.
- Validate the Calibration Framework: Rigorously test the framework with a separate validation set to evaluate its accuracy and reliability. Be prepared to modify the system based on the outcomes of this validation process, ensuring optimal performance.
- Routine Analysis: Following verification, leverage the adjustment framework to analyze unidentified specimens. It is essential to consistently assess the model's performance with quality control items to guarantee ongoing accuracy.
By diligently following these steps, laboratories can seamlessly integrate the NIR equation into their workflows, thereby enhancing their analytical capabilities.
Troubleshoot Common Implementation Issues
When applying the NIR equation, several common issues may arise that warrant attention. Addressing these challenges proactively can significantly enhance the reliability and accuracy of NIR spectroscopy in laboratory settings.
-
Instrument Calibration Errors are a primary concern. If the NIR spectrometer is not calibrated correctly, inaccurate absorbance readings may result. It is crucial to perform regular calibration checks and adjust settings as necessary to ensure optimal performance.
-
Example Variability is another factor that can influence outcomes. Variations in specimen preparation can lead to inconsistent results. To mitigate this, ensure that all specimens are prepared uniformly, and consider implementing standardized procedures for handling.
-
Environmental Factors such as changes in temperature, humidity, or light can also impact NIR measurements. Conducting analyses in controlled environments helps minimize these effects, ensuring more reliable data.
-
Model Performance Issues must not be overlooked. If the calibration model does not yield satisfactory results, it is essential to revisit the calibration set. Confirm that it accurately represents the samples being analyzed, and consider expanding the dataset to improve model robustness.
-
Lastly, Data Analysis Software Errors can hinder accurate results. Ensure that the software used for data analysis is up to date, and that users are adequately trained in its operation. Regular checks for software updates and patches are recommended to maintain functionality.
By addressing these common issues, laboratories can significantly improve their implementations of the NIR equation, leading to more reliable and accurate scientific outcomes.
![]()
Conclusion
Implementing the NIR equation effectively transforms laboratory practices by enhancing analytical precision and efficiency. Understanding the fundamentals of the NIR equation, along with the necessary tools and structured workflows, is essential for laboratories aiming to leverage near-infrared spectroscopy for quantitative analysis. By mastering these components, labs can achieve more reliable results and streamline their processes.
Key insights from this guide highlight the importance of gathering appropriate instruments, such as NIR spectrometers and data analysis software, while also emphasizing the need for rigorous calibration and validation frameworks. Addressing common implementation issues, such as instrument calibration errors and environmental factors, further ensures the accuracy of NIR measurements. By following these steps, laboratories can integrate the NIR equation into their workflows seamlessly and effectively.
In conclusion, the practical applications of the NIR equation extend beyond mere measurement; they represent a significant advancement in analytical methodologies. By adopting best practices and proactively addressing potential challenges, laboratories can unlock the full potential of NIR spectroscopy. Embracing these strategies not only enhances the reliability of scientific outcomes but also positions labs at the forefront of analytical innovation.




