Overview
KCl is a notable electrolyte, as it completely dissociates into potassium (K⁺) and chloride (Cl⁻) ions upon dissolving in water. This characteristic enables it to conduct electricity with remarkable efficiency. Its significance extends beyond mere conductivity; KCl plays a vital role in various applications, such as:
- Electrochemical cells
- pH measurement
The high solubility and ionic strength of KCl are essential for ensuring stable and accurate measurements, thereby underscoring its importance in laboratory settings. Understanding the properties of KCl not only highlights its functionality but also emphasizes the necessity of high-quality scientific instruments in achieving reliable results.
Introduction
Potassium chloride (KCl) transcends its role as a mere seasoning; it stands as a crucial ionic compound with profound implications in both culinary and scientific domains. Its exceptional solubility and capacity to dissociate into potassium and chloride ions establish KCl as an essential component in various applications, particularly as an electrolyte.
This raises a pivotal question: how does KCl's function as an electrolyte enhance its efficacy in laboratory settings and medical applications? A thorough exploration of this multifaceted compound unveils its critical role in:
- Maintaining ionic strength
- Improving measurement accuracy
- Addressing dietary concerns
Thus, KCl emerges as indispensable for those engaged in the intricate interplay between chemistry and health.
Define KCl: Chemical Composition and Properties
Potassium chloride (KCl) stands as a pivotal ionic compound, composed of potassium (K⁺) and anions in a 1:1 ratio. This white crystalline solid is highly soluble in water, making it an essential choice across various applications. With a molecular weight of approximately 74.55 g/mol and a melting point of 770 °C, KCl exhibits remarkable properties that enhance its utility. Its solubility in water reaches about 340 g/L at room temperature, which raises the question of, is KCl an electrolyte? Notably, KCl is scentless and possesses a salty flavor reminiscent of table salt (sodium).
Recent studies, particularly those involving 2% salt and modified KCl in ground beef, underscore KCl's role in enhancing water holding capacity and improving yields in meat processing. This highlights its functional properties that extend beyond traditional uses. Significantly, Nu-Tek's altered salt is recognized as being ionically closest to sodium salt, addressing flavor and yield concerns in meat processing while mitigating the bitter taste often associated with this ingredient.
This versatility emphasizes the , where their solubility and ionic properties are crucial for various analytical procedures. The implications of KCl in both culinary and scientific applications reveal its multifaceted nature, establishing it as a compound of considerable significance.
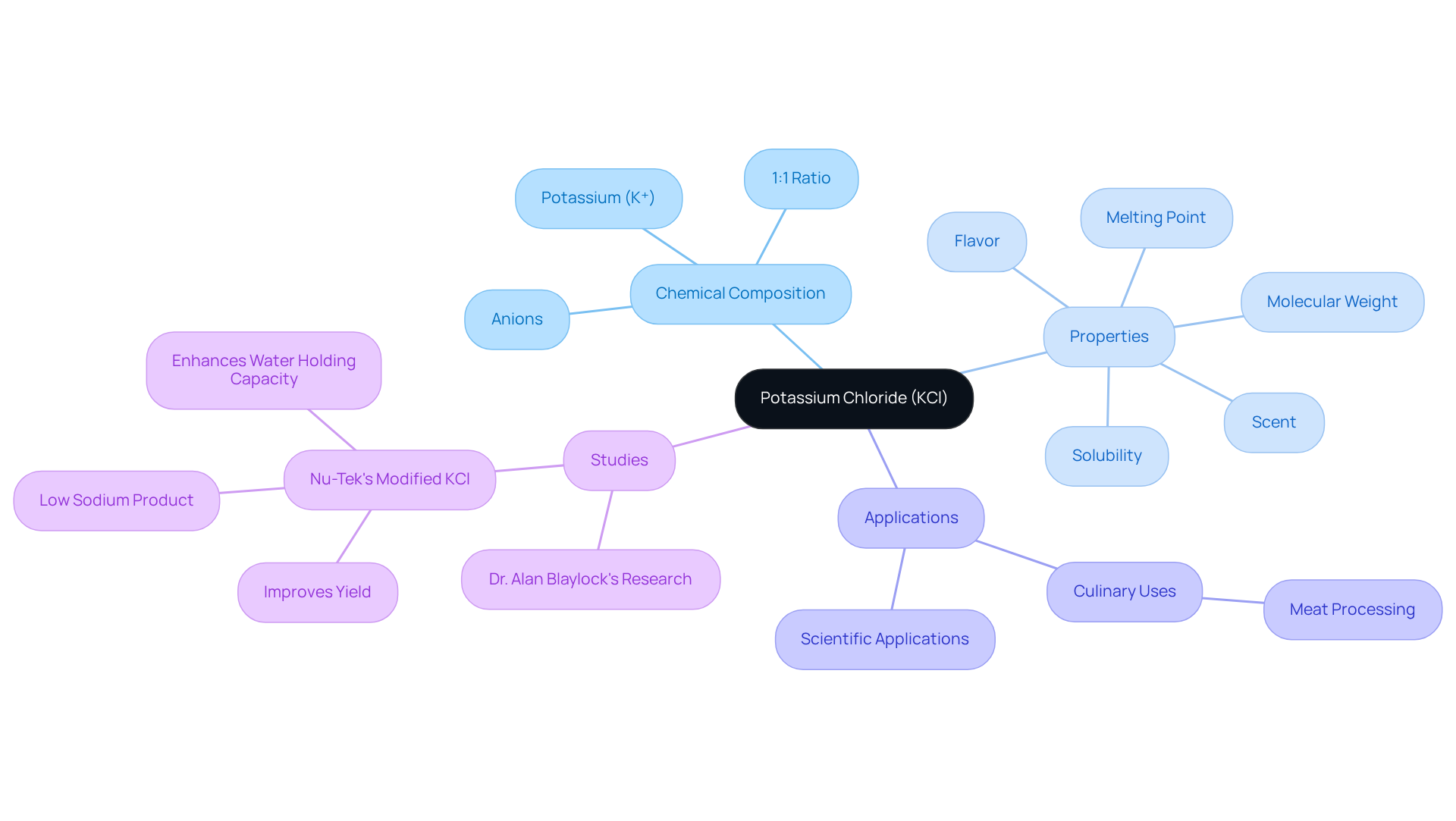
Explore the Importance of KCl in Laboratory Applications
Potassium K (K) stands as a versatile compound extensively utilized in laboratory environments for various applications, notably for its role as an electrolyte, specifically addressing the question of whether KCl is an electrolyte in electrochemical cells. Its high solubility and capacity to dissociate into K+ and Cl- ions render it vital for sustaining ionic strength in solutions, which is critical for . KCl also serves as a key component in buffer solutions and acts as a reagent in numerous chemical reactions. Furthermore, KCl exhibits antimicrobial activity comparable to sodium chloride (NaCl), enhancing its relevance in food safety and preservation contexts.
In electrochemical cells, KCl plays a crucial role by providing a stable ionic environment necessary for reliable measurements. Commonly employed in pH meters, it helps establish a stable reference potential, ensuring precision in readings. Recent developments underscore KCl's effectiveness in reducing sodium intake in food products, showcasing its potential as a safer alternative to traditional salt (NaCl), which is linked to hypertension.
Statistically, KCl can reduce the amount of salt in foods by up to 25%, making it an invaluable tool in dietary management. The average intake of this mineral in the United States is approximately 72 mmol/d, with recommendations suggesting an increase of 30-45 mmol/d for improved health outcomes. KCl is also essential in healthcare, particularly in managing hypokalemia, where it aids in preventing significant loss of this mineral. However, it is important to acknowledge that there have been recalls of potassium chloride capsules due to potential release issues, highlighting safety concerns associated with its use.
Case studies have demonstrated the successful encapsulation of KCl in sustained release dosage forms, enhancing its therapeutic effects while minimizing potential side effects. This innovation emphasizes KCl's significance not only in laboratory environments but also in clinical uses, where maintaining electrolyte balance is crucial for patient care. Overall, KCl's unique characteristics and various applications solidify its status as , making one wonder if KCl is an electrolyte in both research and medical fields.
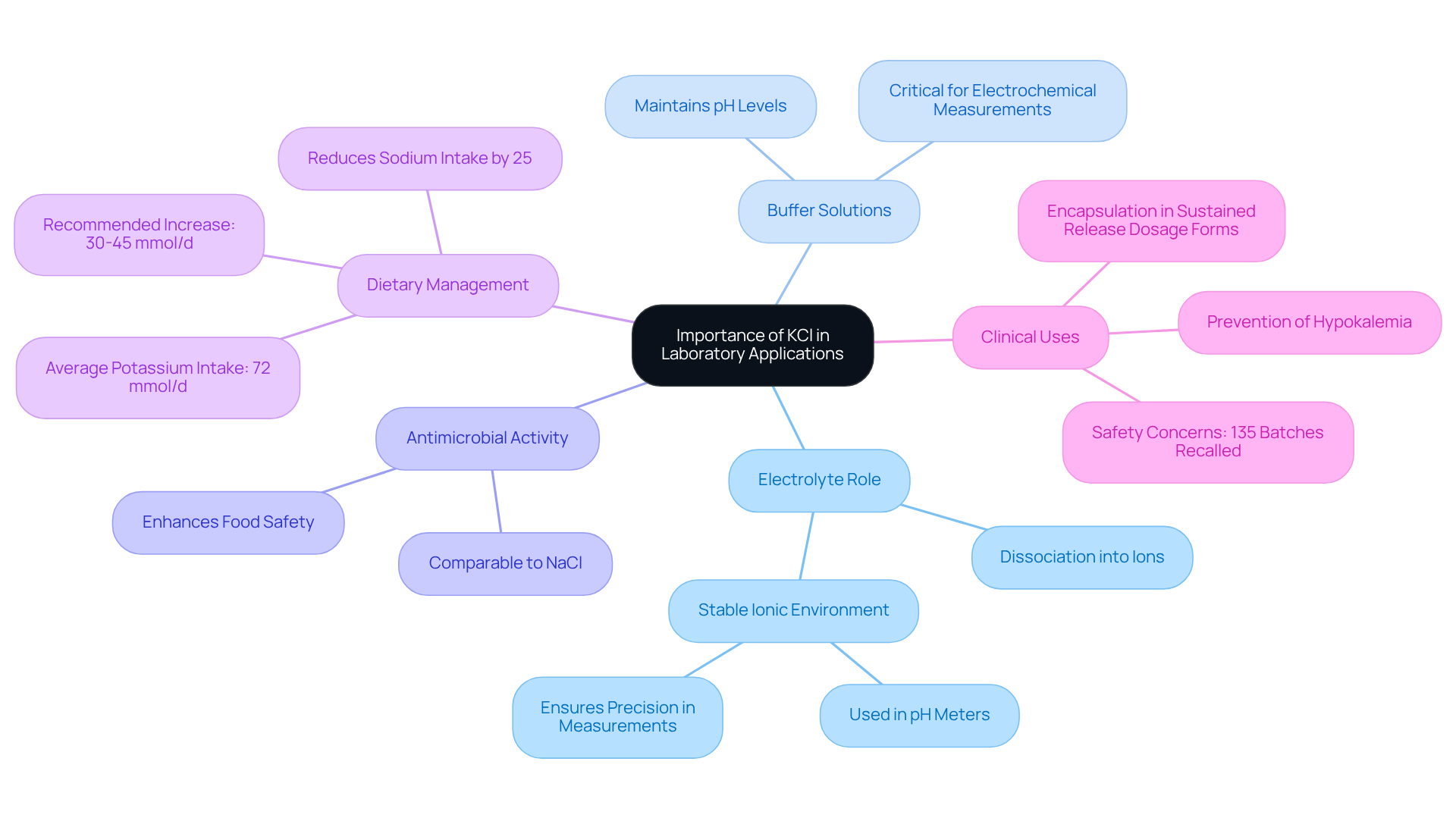
Identify Key Characteristics of KCl as an Electrolyte
KCl is an electrolyte that is recognized as a strong electrolyte due to its complete dissociation into potassium (K+) and chloride (Cl-) ions when dissolved in water. This complete ionization is crucial, as it enables [KCl solutions](https://jmscience.com) to conduct electricity effectively, leading to the inquiry: is KCl an electrolyte suitable for various electrochemical applications?
The conductivity of KCl solutions is significantly influenced by concentration and temperature; notably, higher concentrations result in increased conductivity. For example, a typical KCl solution concentration is 0.75% (w/v), commonly utilized in diverse laboratory settings. This characteristic is particularly important in contexts such as electrochemical cells, where a stable ionic environment is essential for precise measurements and reliable outcomes.
For further assistance and resources on KCl solutions, JM Science offers , including how-to videos and resource libraries, empowering laboratory managers to access valuable information that enhances their use of KCl in experiments.
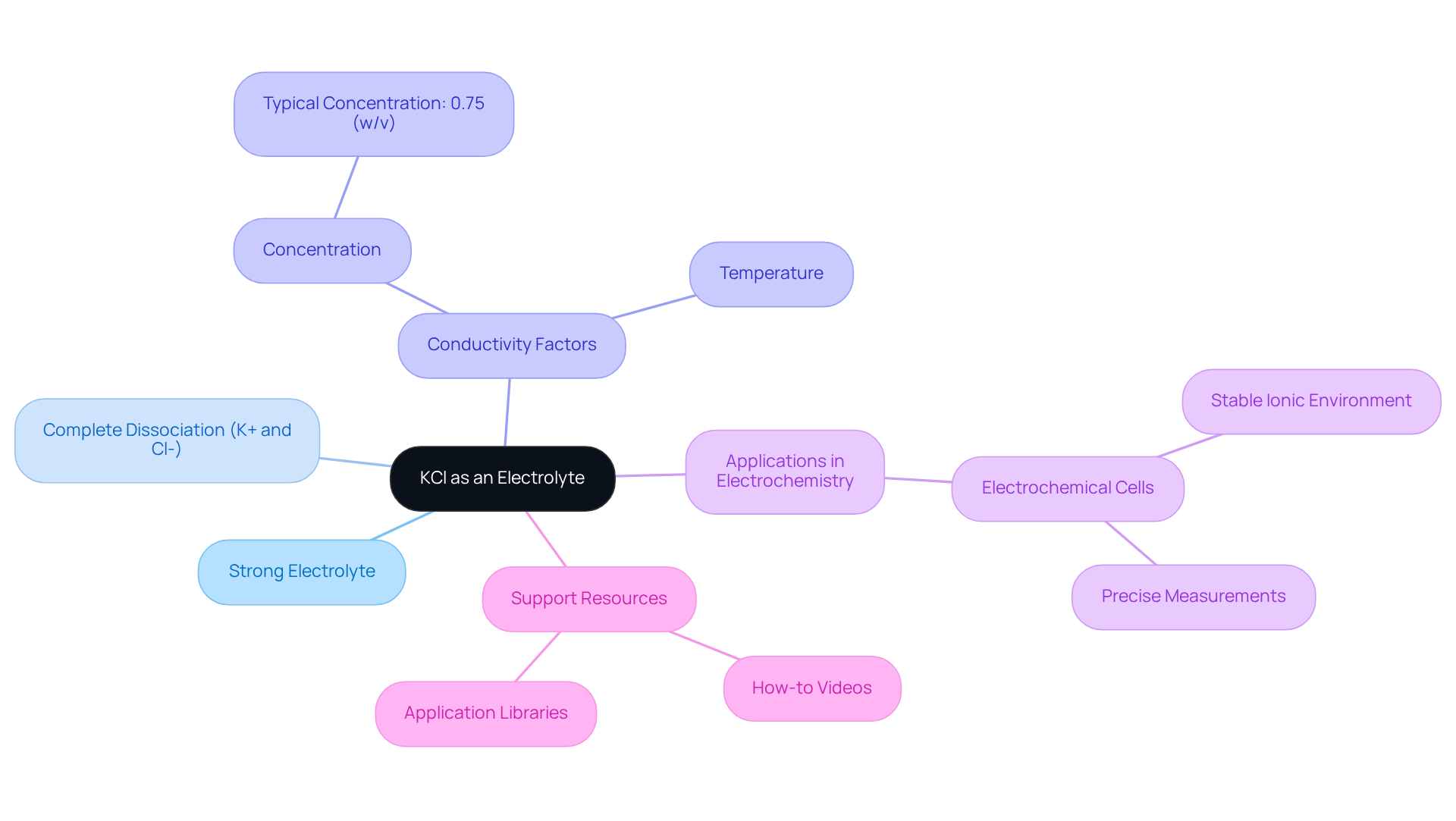
Examine the Role of KCl in pH Measurement and Electrochemical Analysis
KCl serves a critical function in pH meters as a filling solution for reference electrodes. Its neutral pH and are vital for sustaining a stable reference potential, which is essential for obtaining accurate pH readings. Moreover, KCl solutions are frequently utilized in electrochemical analysis, including potentiometry and voltammetry, which raises the question, is KCl an electrolyte, as they facilitate ion movement and enhance solution conductivity. The incorporation of KCl in these applications ensures that measurements are reliable and reproducible, solidifying its status as a fundamental component in both academic and industrial laboratories.
Recent research underscores the significant influence of KCl on pH measurements. For example, spiking with 0.01, 0.10, and 0.19 KCl yields pH errors of 0.02, 0.06, and 0.09 pH units, respectively, when compared to unspiked samples. Such discrepancies emphasize the necessity for meticulous consideration when employing KCl in low ionic strength solutions, as even minor alterations can lead to substantial variations in pH readings.
Case studies further demonstrate the importance of KCl in preserving measurement accuracy. For instance, experiments reveal that while KCl additions can enhance measurement precision, they may simultaneously compromise accuracy, particularly in samples with low solute content. This dual effect highlights the imperative for laboratories to weigh the advantages of KCl against the potential for induced errors in pH measurements. As R. C. Metcalf aptly stated, "For these reasons we do not recommend KCl additions or stirring of water samples of low ionic strength during studies emphasizing the accuracy of pH measurement."
In summary, the question of whether is KCl an electrolyte highlights its indispensable role in electrochemical analysis, as it provides the necessary conditions for accurate and consistent measurements across a variety of applications.
Conclusion
Potassium chloride (KCl) stands out as a strong electrolyte, characterized by its complete dissociation into potassium (K⁺) and chloride (Cl⁻) ions upon dissolving in water. This fundamental property underpins its extensive applications in both laboratory and clinical settings, where maintaining ionic strength is essential for accurate measurements and effective treatments.
The article provides key insights into KCl's versatility. Its high solubility not only enhances its conductivity in electrochemical cells but also supports its role in pH measurement, buffer solutions, and food preservation. Moreover, KCl serves as a valuable alternative in dietary management by reducing sodium intake while supplying essential potassium. The discussion further emphasizes KCl's importance in laboratory practices, highlighting the necessity of careful consideration regarding its impact on measurement accuracy.
In summary, KCl emerges as a compound of significant relevance in both scientific research and everyday applications. Its role as an electrolyte transcends chemical properties, influencing health, safety, and precision across various fields. Understanding KCl's characteristics and applications empowers individuals and professionals to utilize this compound effectively, whether in the lab, the kitchen, or the clinic.




