Overview
Mastering chiral HPLC in your laboratory hinges on several key strategies:
- Selecting the appropriate chiral column
- Optimizing mobile phase conditions
- Validating methods
- Ensuring continuous training for staff
These strategies are not merely recommendations; they are essential practices that significantly enhance separation efficiency, compliance, and overall laboratory performance. Case studies and expert insights underscore the profound impact of proper technique implementation and ongoing education in achieving reliable analytical results. By prioritizing these strategies, laboratories can position themselves at the forefront of analytical excellence, paving the way for improved outcomes and sustained success.
Introduction
In the realm of analytical chemistry, the significance of chiral chromatography is paramount. As industries increasingly depend on precise separation techniques to guarantee the quality and efficacy of pharmaceuticals, the selection of the appropriate chiral column and the optimization of mobile phase conditions have become crucial.
This article explores the intricacies of chiral HPLC, detailing essential strategies for:
- Method validation
- Temperature control
- The selection of detection methods
It underscores the importance of meticulous documentation and ongoing training for laboratory staff, while also addressing common challenges encountered during method development.
By collaborating with experts and remaining informed about technological advancements, laboratories can enhance their chiral HPLC processes. This ultimately leads to improved analytical outcomes and compliance with industry standards.
Choose the Right Chiral Column for Your Application
Selecting the appropriate optical column for chiral HPLC applications necessitates a comprehensive understanding of the specific characteristics of the compounds being analyzed. Key factors in chiral HPLC—such as chemical structure, polarity, and the nature of the enantiomers—significantly influence separation efficiency. Among the most popular types of asymmetric columns utilized in chiral HPLC are:
- Polysaccharide-based
- Protein-based
- Macrocyclic glycopeptide columns
Notably, polysaccharide columns are particularly favored for their versatility across a wide range of solvents and conditions, rendering them suitable for various applications.
To ensure optimal results, consulting the manufacturer's guidelines is essential, along with conducting preliminary tests. This approach allows for the identification of the best column fit for your specific application. JM Science Inc., recognized for its dedication to innovation in scientific instrumentation, offers a variety of high-quality columns that can satisfy diverse laboratory requirements. Their partnerships with leading brands like Agilent Technologies and Thermo Fisher Scientific further enhance the reliability of their products.
Staying informed about current trends in asymmetric chromatography can significantly enhance decision-making. Recent advancements in column technology have led to improved resolution and faster analysis times, which are critical in high-throughput environments. As noted by Daicel, a chromatography specialist, 'Assistance providing stationary phases for large-scale applications' is essential for achieving optimal separation outcomes in chiral HPLC.
Practical instances illustrate the effective choice of asymmetric columns in research facilities, demonstrating how chiral HPLC methods can yield notable enhancements in separation results. For instance, a recent case study involving JM Science's asymmetric columns showcased improved resolution in the separation of complex mixtures, underscoring the significance of professional insights on column selection. As the field of analytical chemistry evolves, the necessity for continuous learning and adaptation in laboratory practices becomes increasingly evident.
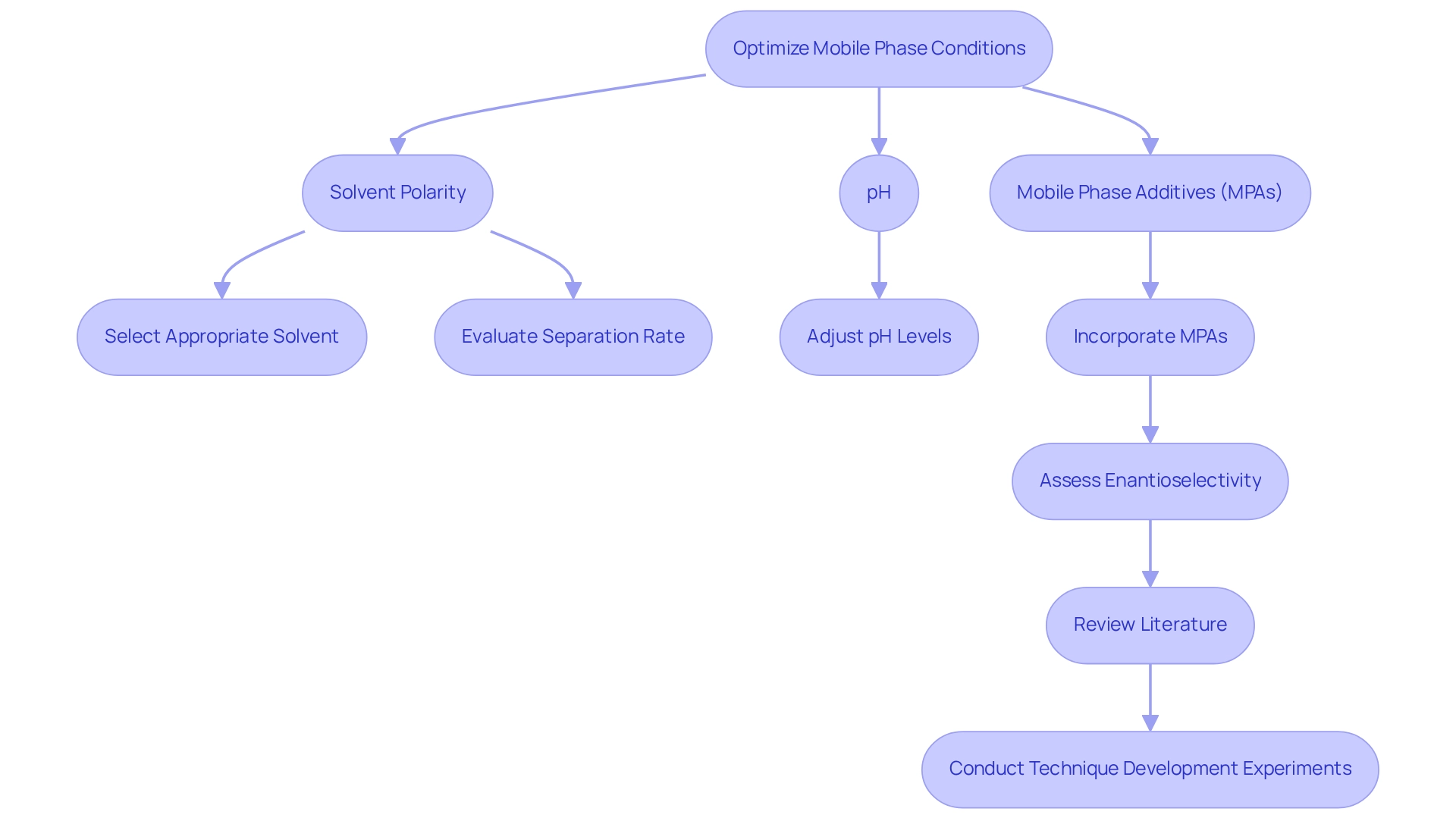
Optimize Mobile Phase Conditions for Enhanced Separation
Optimizing mobile phase conditions is crucial for achieving successful enantioselective separations in chiral HPLC. Key factors to consider include solvent polarity, pH, and the incorporation of mobile phase additives (MPAs). Recent studies underscore the significance of solvent selections, highlighting that the use of MPAs can greatly enhance enantioselectivity, making them indispensable tools in asymmetric HPLC applications.
The impact of solvent polarity on separation success rates is profound. Goel et al. demonstrated that optimized mobile phase conditions could facilitate quicker separations of labetalol in under 15 minutes. Additionally, a confirmed technique for isolating guaifenesin enantiomers and ambroxol emphasizes the necessity of adopting more sustainable approaches in chiral HPLC.
Case studies further illustrate the effectiveness of mobile phase optimization. A notable study developed a cellulose tris(3-chloro-4-methylphenylcarbamate) stationary phase (CSP) for the simultaneous determination of eslicarbazepine acetate and its metabolites, showcasing the approach's applicability in in vitro studies of stereoselective metabolism. Another investigation employed packed column CEC–MS to enhance separation capabilities for complex pharmaceutical mixtures, achieving high-resolution separations of stereoisomers.
As Scriba et al. astutely observed, "it should be kept in mind that very often the selectors also display chemoselectivity because binding thermodynamics also differ for structurally related or unrelated compounds." To remain at the forefront of chiral HPLC, it is imperative to continually review the literature and conduct technique development experiments. This proactive strategy will facilitate the identification of the most effective mobile phase conditions tailored to specific separation requirements, ultimately advancing progress in pharmaceutical analysis.
Validate Your Method to Ensure Compliance and Reliability
Validating chiral HPLC techniques is essential for ensuring compliance and reliability within pharmaceutical environments. This process must include comprehensive assessments of:
- Specificity
- Accuracy
- Precision
- Linearity
- Robustness
Adhering to the ICH Q2(R1) guidelines is paramount, as these recommendations establish a solid framework for the validation of analytical procedures, fostering both consistency and regulatory compliance. Meticulous documentation of all validation procedures is vital, as it facilitates compliance during audits and inspections, thereby enhancing the reliability of your facility's results.
Moreover, frequent reassessment of techniques is crucial, allowing organizations to adapt to evolving regulatory demands and advancements in scientific practices. The case study titled 'Significance of ICH Q2(R1) Guideline' underscores the importance of these guidelines in maintaining regulatory compliance and improving the reliability of analytical results derived from chiral HPLC in the pharmaceutical sector. Statistics indicate that adherence to ICH Q2(R1) guidelines significantly boosts validation compliance rates in pharmaceutical laboratories, highlighting the importance of these practices in achieving regulatory success. This reinforces the necessity for laboratories to remain informed about current regulatory requirements and to continuously validate their methods.
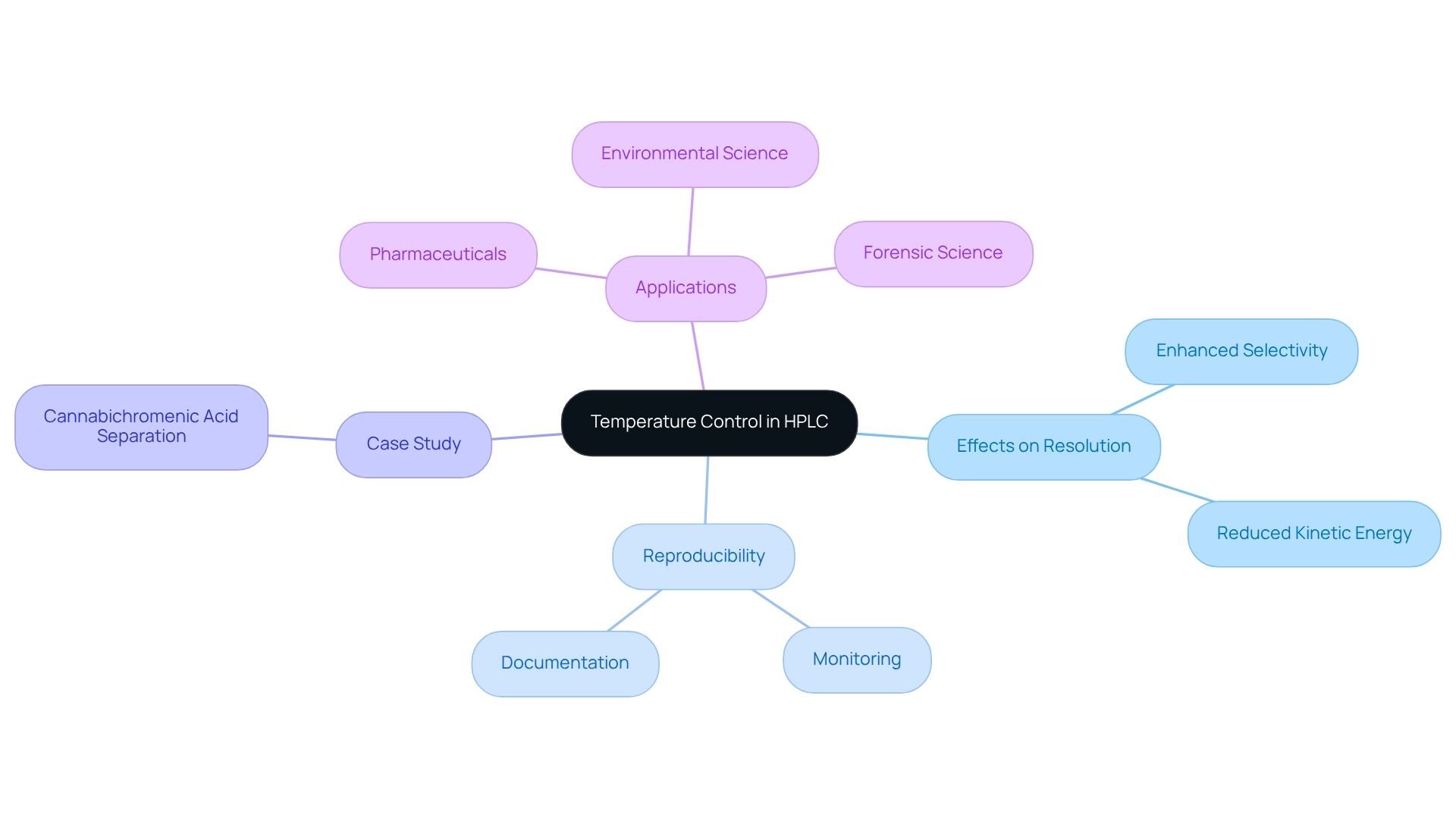
Control Temperature for Improved Resolution and Reproducibility
Temperature significantly influences the interaction between the analyte and the optical stationary phase in high-performance liquid chromatography (HPLC). By reducing temperatures, selectivity can be enhanced as the kinetic energy of molecules decreases, often leading to improved resolution in asymmetric separations. Therefore, implementing robust temperature control systems within your HPLC setup is not just beneficial but essential for maintaining consistent analytical conditions.
Regular monitoring and documentation of temperature variations are critical for ensuring reproducibility of results. Expert insights suggest that optimizing temperature can dramatically impact resolution in chiral HPLC. For example, a case study involving chiral HPLC with the CHiRAL 5A column illustrated successful separation of Cannabichromenic Acid (CBCA) enantiomers, demonstrating how precise temperature management can elevate analytical outcomes.
Furthermore, statistics reveal that mastering chromatographic factors, including temperature control, plays a significant role in advancements within pharmaceuticals and environmental science, with over 7,900 applications utilizing COSMOSIL columns. By prioritizing temperature optimization in laboratory chromatography setups, laboratories can achieve substantial improvements in both resolution and reproducibility. In forensic science, effective temperature regulation in HPLC is vital for accurately analyzing evidence and detecting substances, underscoring its practical implications in real-world scenarios.
Select Appropriate Detection Methods for Accurate Analysis
In chiral HPLC, typical detection techniques encompass UV-Vis, fluorescence, and circular dichroism (CD) detection. Each method presents unique advantages; notably, chiral HPLC detection excels in differentiating enantiomers due to its heightened sensitivity to optical activity. This capability is vital for achieving precise enantiomeric separation, particularly in pharmaceutical applications where chiral HPLC is indispensable for ensuring drug quality. A recent case study underscored a high-throughput chiral HPLC analysis that improved resolution and efficiency for the simultaneous enantioseparation of multiple profens, illustrating the effectiveness of advanced detection techniques in real-world scenarios.
Moreover, thermal regulation in HPLC plays a critical role in maintaining stable retention times and accurate separation, further emphasizing the importance of selecting appropriate detection techniques. When determining a detection approach, it is crucial to evaluate sensitivity, selectivity, and compatibility with specific analytes. Chiral HPLC detection offers substantial advantages, including enhanced sensitivity for asymmetric compounds and the capability for real-time monitoring of enantiomeric purity. As the pharmaceutical industry increasingly prioritizes effective impurity control strategies to enhance drug quality and consumer trust, the choice of detection method becomes paramount. Analytical chemists have recognized that CD detection is particularly effective for enantiomers, highlighting the significance of chiral HPLC in modern asymmetric analysis.
Marco Antônio Garcia dos Santos remarked, "A semipreparative column filled with microcrystalline cellulose triacetate (MCTA) was utilized to separate ketamine enantiomers through high-performance liquid chromatography." This statement reinforces the effectiveness of specific methods in asymmetric liquid chromatography.
By staying informed about current trends and technological advancements in detection techniques, laboratories can refine their optical separation processes and uphold the highest standards of precision in their analyses. A comparative analysis of detection techniques for chiral HPLC indicates that while UV-Vis and fluorescence possess their merits, CD detection is frequently favored for its superior sensitivity and real-time monitoring capabilities.
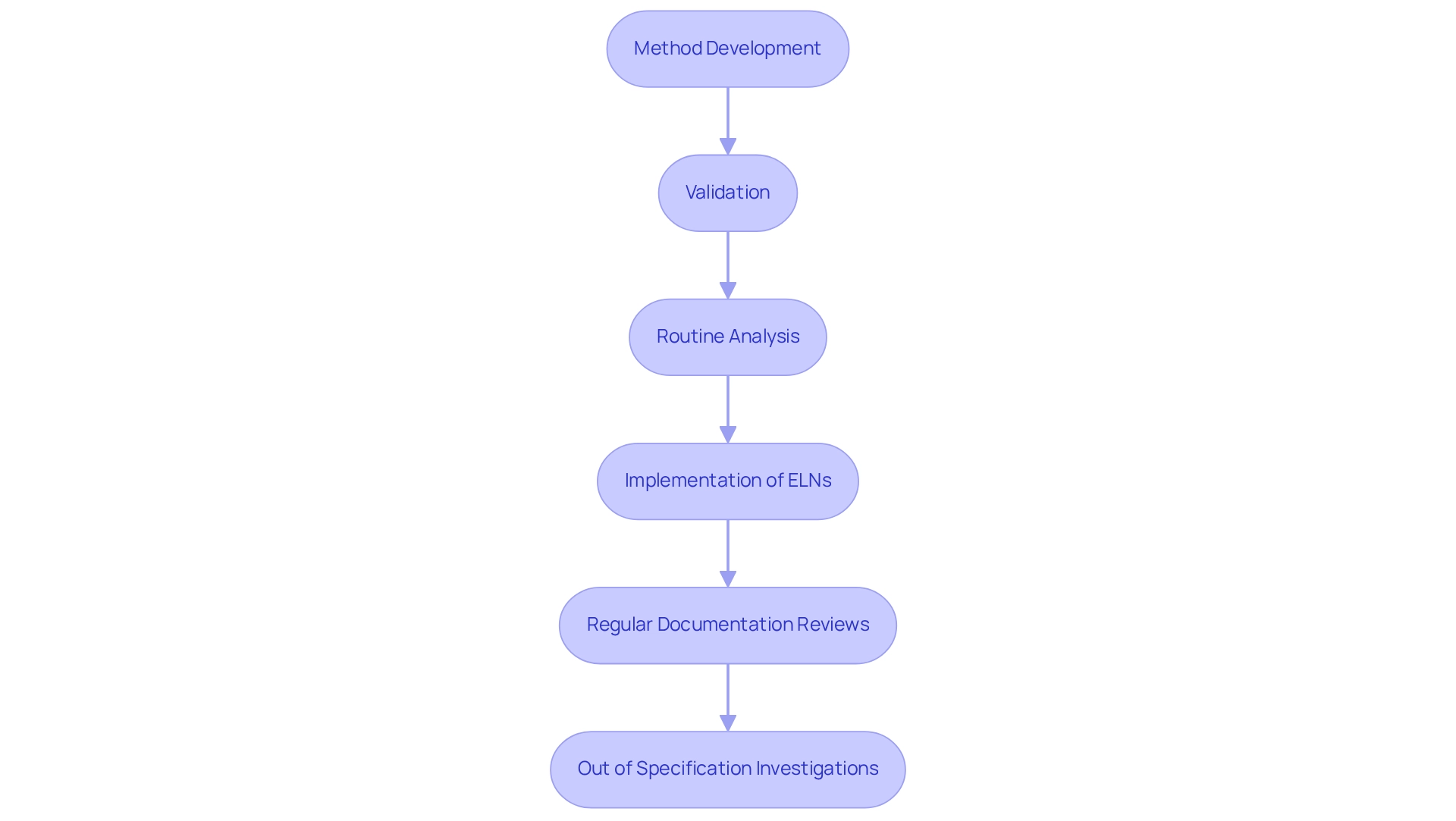
Document Your Process for Reproducibility and Compliance
Creating a comprehensive documentation system is essential for capturing every aspect of the chiral HPLC process, encompassing method development and validation through to routine analysis. The implementation of electronic lab notebooks (ELNs) can significantly streamline data entry, ensuring that all records are both accessible and well-organized. Regular reviews of documentation practices are vital to align with Good Laboratory Practices (GLP) and uphold compliance with industry standards.
Statistics indicate that efficient documentation methods in asymmetric liquid chromatography facilities enhance reproducibility, a critical component in achieving reliable analytical outcomes. As highlighted, "chromatography is a gateway to understanding the complexities of matter," underscoring the significance of thorough documentation in this discipline. For instance, a case study titled "Rapid Analysis with Chromatography" demonstrated that effective documentation not only accelerates decision-making in clinical settings but also supports quality control processes.
Laboratory managers have recognized the transformative impact of ELNs on compliance, asserting that "the quality of an analytical result begins with the sample," which emphasizes the necessity for meticulous documentation. By adopting ELNs, research facilities can guarantee that their documentation practices meet the stringent demands of scientific inquiry, ultimately fostering a culture of accuracy and integrity within their chiral HPLC processes. Furthermore, the importance of scientifically sound Out of Specification (OOS) investigations cannot be overstated, as they are crucial for ensuring data integrity, thereby reinforcing the need for robust documentation practices.
Invest in Continuous Training for Laboratory Staff
To ensure that research personnel remain proficient in the latest chiral high-performance liquid chromatography techniques, regular training sessions are essential. A strategic implementation of workshops, online courses, and hands-on training significantly enhances skill sets. Furthermore, encouraging attendance at industry conferences and seminars keeps staff informed about emerging trends and best practices. This commitment to ongoing education not only fosters innovation but also leads to improved performance in the laboratory.
Research indicates that continuous training programs can bolster staff competency, ultimately contributing to more accurate and efficient analyses. Notably, studies reveal that training effectiveness can elevate staff performance by as much as 30%. As the HPLC market evolves—particularly in the Asia Pacific region, projected to experience the fastest revenue growth—staying ahead through effective training becomes increasingly vital for maintaining a competitive edge.
Industry leaders emphasize the importance of ongoing training, asserting that "investing in staff education is essential for adapting to technological progress and ensuring adherence to ethical standards in practice." Additionally, as research facilities incorporate more advanced technologies, it is crucial to address ethical implications, such as data privacy and AI-driven analysis, through continuous training.
Current trends indicate a shift towards more interactive and technology-driven training programs, further enhancing the learning experience for laboratory staff.
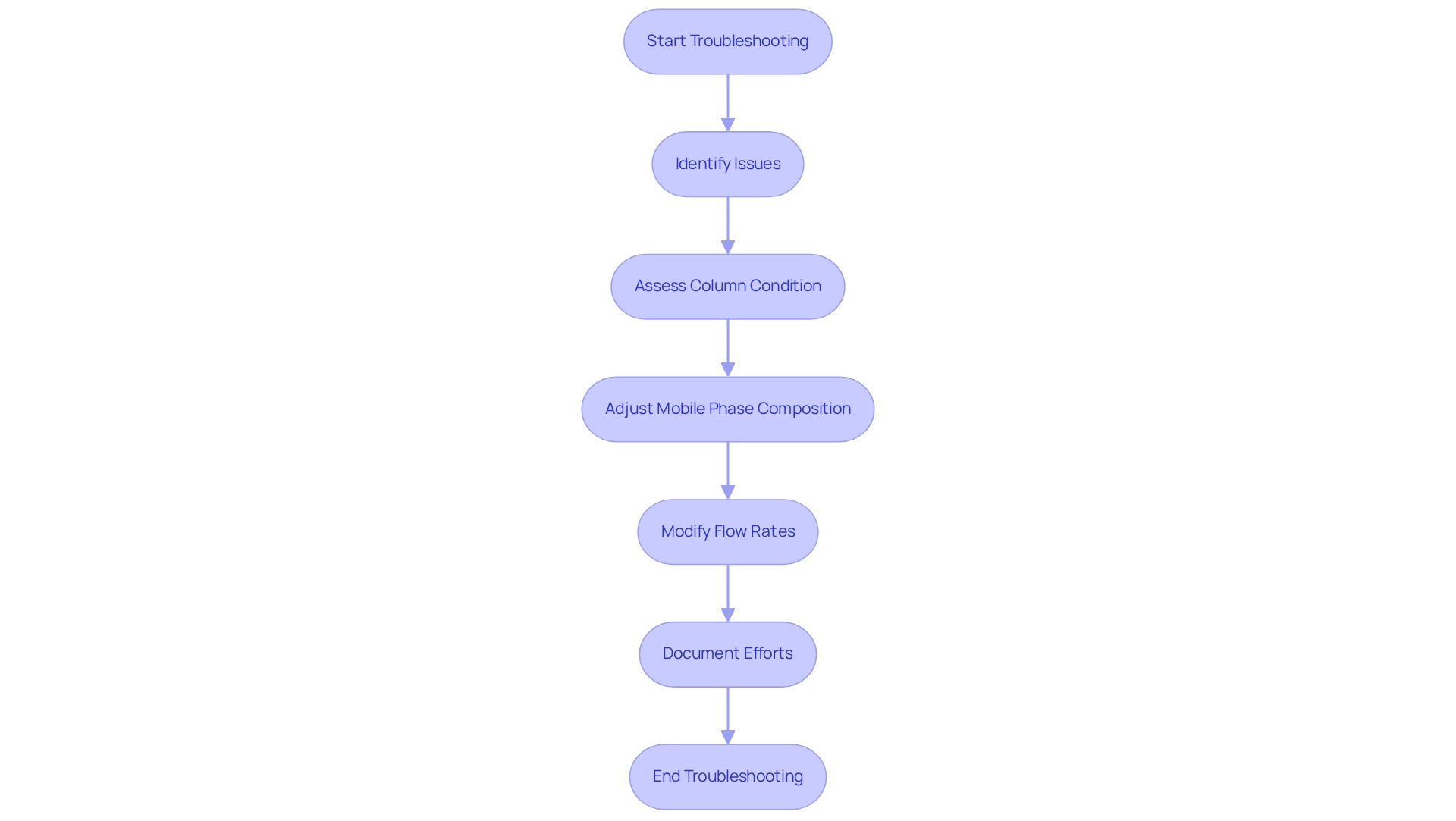
Troubleshoot Common Issues in Chiral HPLC Development
Common issues encountered in chiral HPLC include poor resolution, peak tailing, and inconsistent retention times. Addressing these challenges necessitates a thorough assessment of the chiral HPLC column's condition, as the history of a chiral HPLC column significantly impacts the reproducibility of chiral separations. Proper maintenance is crucial; neglecting this aspect can lead to peak shape issues and memory effects that persist for thousands of column volumes.
To effectively troubleshoot these problems in chiral HPLC, consider adjusting the mobile phase composition and flow rates. While isocratic techniques may seem easier, they often result in inadequate elution of sample components, hindering enantiomer quantification. A case study titled "Challenges in Enantiomer Quantification" underscores that the choice of column and separation method is pivotal in achieving accurate results. This highlights the importance of selecting appropriate stationary phases to avoid complications such as additive memory effects.
Engaging with the analytical community through forums and discussions offers valuable insights and shared experiences that can enhance problem-solving capabilities. As chromatography expert Sirshendu Roy noted, "This document provides an overview of high performance liquid chromatography (HPLC)," emphasizing the necessity for a comprehensive understanding of HPLC principles.
Documenting all troubleshooting efforts is essential for refining future practices. This systematic approach not only addresses current problems but also deepens comprehension of the underlying causes, ultimately leading to improved results in separations. Statistics indicate that systematic troubleshooting methods can significantly enhance success rates in resolving common issues in chromatography. By leveraging expert knowledge and proven troubleshooting techniques, research facilities can optimize their HPLC processes and achieve more reliable analytical outcomes.
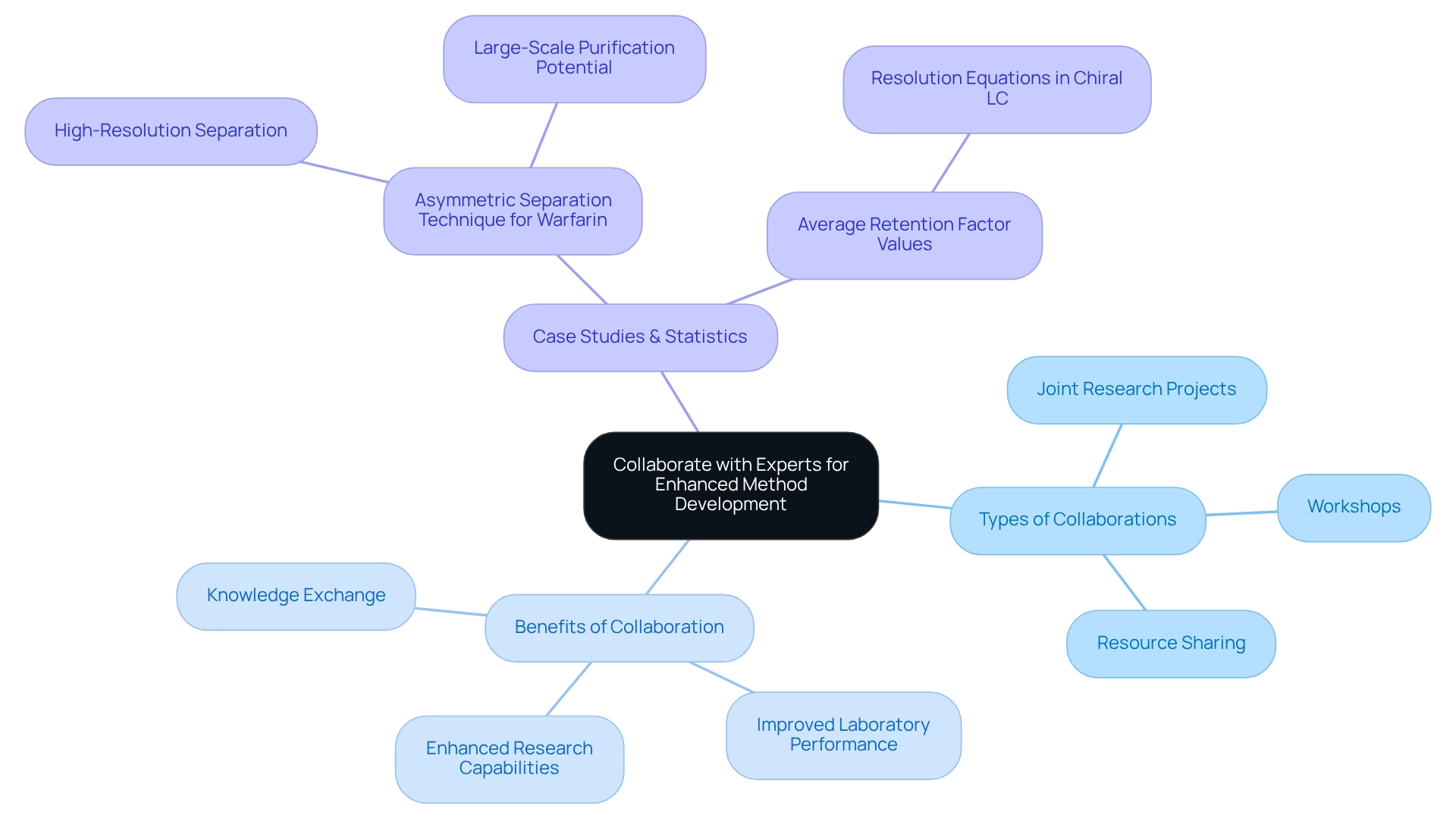
Collaborate with Experts for Enhanced Method Development
Interacting with chromatography specialists and advisors is essential for the advancement of sophisticated technique development strategies. Collaborations can manifest in various forms, including:
- Joint research projects
- Workshops
- Resource sharing
All of which significantly enhance research capabilities. Engaging at conferences and within professional associations often leads to fruitful collaborations that propel advancements in chiral HPLC techniques. Establishing advisory boards composed of industry specialists provides vital direction in development efforts. Such collaborations not only facilitate knowledge exchange but also contribute to improved laboratory performance metrics.
For instance, a case study on the creation of an asymmetric separation technique for warfarin enantiomers demonstrates how expert consultations can yield high-resolution separations, underscoring the potential for large-scale purification and process development. This scenario emphasizes the critical role of specialist involvement in achieving favorable outcomes.
Furthermore, advancements in asymmetric chromatography, such as the removal of the resolution requirement for producing calibration curves in chiroptical detection, illustrate the evolving landscape of technique development. Understanding mass transport and binding equilibria is crucial for developing predictive chromatography models, further highlighting the complexities inherent in this field.
Statistics indicate that collaborative efforts in method development for asymmetric liquid chromatography frequently yield superior results, with numerous laboratories reporting enhanced efficiency and precision in their analyses. Specifically, average retention factor values can be assessed using resolution equations in enantioselective liquid chromatography, providing quantitative validation for claims of improved performance.
Industry leaders, including Francesco Gasparrini, stress the importance of stationary phases designed for chiral HPLC to facilitate the transition towards enantioselective applications using ultrahigh-performance liquid chromatography. By leveraging the expertise of chromatography specialists, laboratories can remain at the forefront of analytical progress, ensuring they meet the evolving demands of the pharmaceutical sector.
Stay Informed on Advancements in Chiral HPLC Technologies
To remain at the forefront of specialized liquid chromatography technologies, it is essential to regularly engage with scientific literature, attend industry conferences, and participate in webinars. Subscribing to specialized journals and newsletters guarantees access to the latest updates on innovative techniques, cutting-edge equipment, and best practices in the field. Notably, comprehensive reports of 183 pages provide in-depth information that can significantly enhance your understanding. Furthermore, participation in online discussions and professional networks yields valuable insights into emerging trends and innovations in asymmetric chromatography.
Recent advancements in asymmetric stationary phases have markedly improved the selectivity and efficiency of enantiomeric columns, propelling growth in this sector. JM Science Inc. offers a diverse selection of high-quality HPLC solutions, including high-performance liquid chromatography columns and accessories, which are vital for efficient enantiomer analysis. As the Market Statsville Group observes, the increasing demand for optical analysis tools arises from pharmaceutical research into complex diseases and the development of targeted therapies, including personalized medicine applications. Staying informed about these developments is crucial. Engaging with case studies, such as 'Advancements in Stationary Phases,' provides practical examples of how these innovations are applied in real-world settings, further enriching your understanding and application of asymmetric chromatography. Additionally, awareness of strategic partnerships among key market players, including JM Science, aimed at developing advanced technologies underscores the collaborative efforts driving innovation and growth in chiral HPLC technologies.
Conclusion
The exploration of chiral chromatography underscores its critical role in the pharmaceutical industry, highlighting the necessity for meticulous attention to various aspects of the chiral HPLC process.
- Selecting the right chiral column necessitates a deep understanding of compound characteristics.
- Optimizing mobile phase conditions can significantly enhance separation efficiency.
- Furthermore, method validation is essential for compliance and reliability, ensuring that laboratories adhere to industry standards and regulatory guidelines.
Moreover, controlling temperature and selecting appropriate detection methods are pivotal in achieving accurate analyses and reproducibility.
- Comprehensive documentation practices not only improve reproducibility but also support compliance with Good Laboratory Practices.
- Continuous training for laboratory staff is vital to keep pace with evolving technologies and methodologies, fostering a culture of innovation and excellence.
Collaborating with experts and staying informed about advancements in chiral HPLC technologies can further boost laboratory capabilities.
By adopting these strategies, laboratories can enhance their chiral HPLC processes, leading to improved analytical outcomes that meet the rigorous demands of the pharmaceutical landscape. Ultimately, a commitment to quality, continuous learning, and collaboration will pave the way for advancements in chiral chromatography, ensuring that laboratories can effectively contribute to the development of safe and effective pharmaceutical products.




