Overview
The article serves as an authoritative guide on establishing an automatic titration system in laboratories, detailing the essential equipment, step-by-step setup procedures, and troubleshooting strategies. It underscores the advantages of automatic titration, including heightened accuracy, efficiency, and safety, all supported by specific examples and thorough instructions designed to ensure optimal performance in analytical processes.
Introduction
Automatic titration represents a revolutionary advancement in analytical chemistry, capturing the attention of laboratories by offering the precision and efficiency essential for accurate solution concentration measurements. This guide delves into the fundamental principles and benefits of automatic titration, generating interest by highlighting its potential to significantly enhance laboratory workflows while minimizing human error.
Furthermore, it is important to acknowledge that, as with any sophisticated system, challenges can arise during setup and operation. What are the common pitfalls that can hinder optimal performance, and how can they be effectively addressed? By exploring these aspects, we aim to foster a deeper understanding of automatic titration's role in modern laboratories.
Understand Automatic Titration: Principles and Benefits
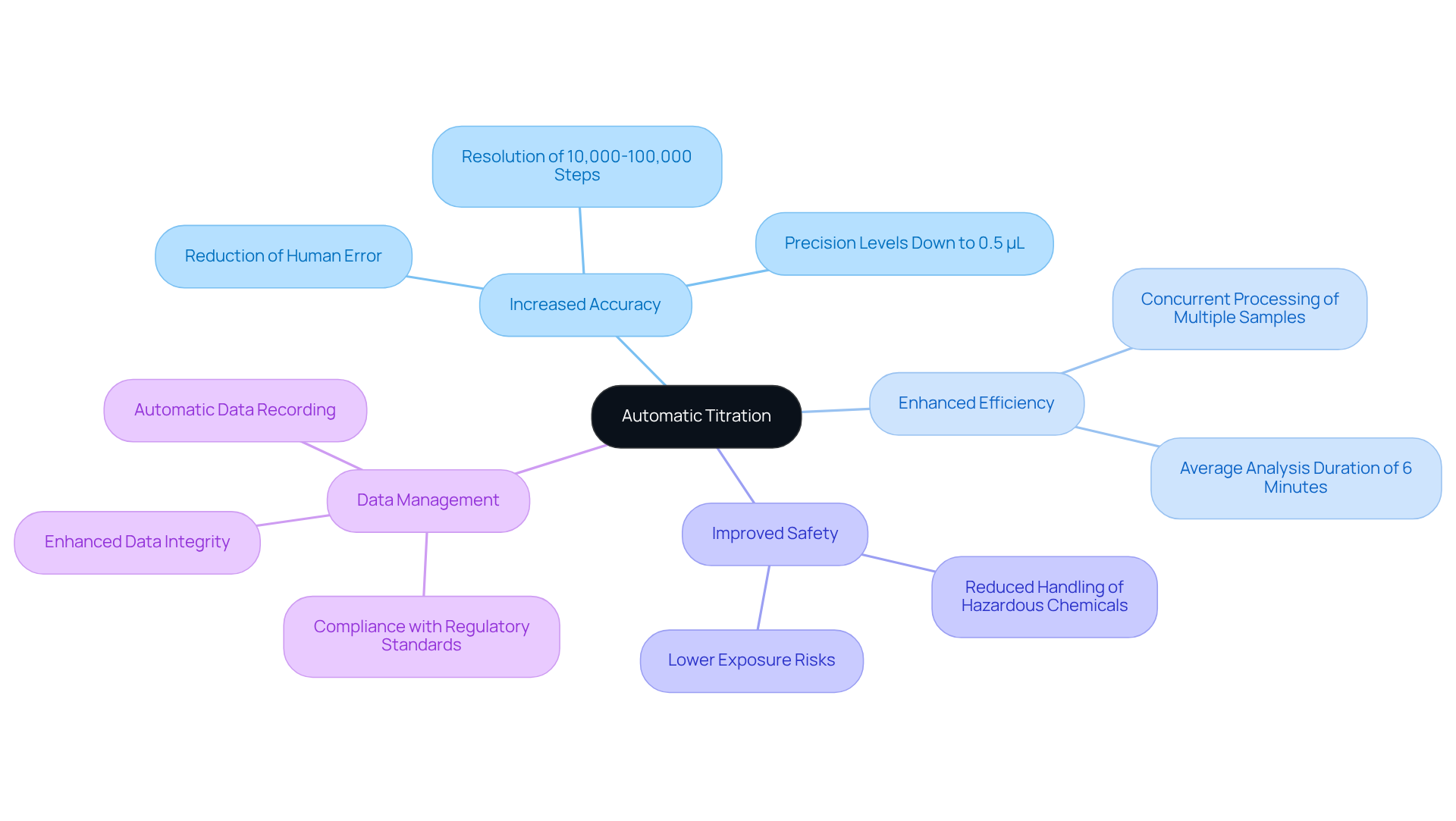
Automatic measurement signifies a substantial leap forward in analytical techniques, automating the determination of solution concentrations with remarkable precision. By employing a motor-driven burette, these systems dispense titrant in controlled increments, facilitating accurate endpoint detection through methods such as potentiometric or photometric measurements. The advantages of automatic titration are extensive:
- Increased Accuracy: Automated systems significantly diminish human error, resulting in more reliable and consistent outcomes. For example, contemporary auto-titrators can achieve a resolution of 10,000-100,000 steps, enabling precision levels down to 0.5 μL.
- Enhanced Efficiency: The enhanced efficiency of automatic titration allows for the concurrent processing of multiple samples, markedly increasing laboratory throughput. The average analysis duration for each automated procedure is approximately 6 minutes, thereby streamlining workflows.
- Improved Safety: Improved safety is ensured by automatic titration, which reduces the necessity for direct handling of hazardous chemicals, consequently lowering exposure risks. This aspect is especially critical in environments where safety is of utmost importance.
- Data Management: Many automated dosing systems are equipped with software for automatic titration that automatically records data, ensuring compliance with regulatory standards and enhancing data integrity. This is essential in the pharmaceutical sector, where accuracy is governed by stringent protocols.
Real-world applications underscore the effectiveness of these systems. For instance, the transition to automated potentiometric analysis has led to notable improvements in precision and efficiency, minimizing potential human error and reducing the time spent on manual procedures. By integrating automated measurement into laboratory workflows, personnel can elevate both the quality of their analyses and the safety of their operations.
Gather Required Equipment and Materials for Automatic Titration
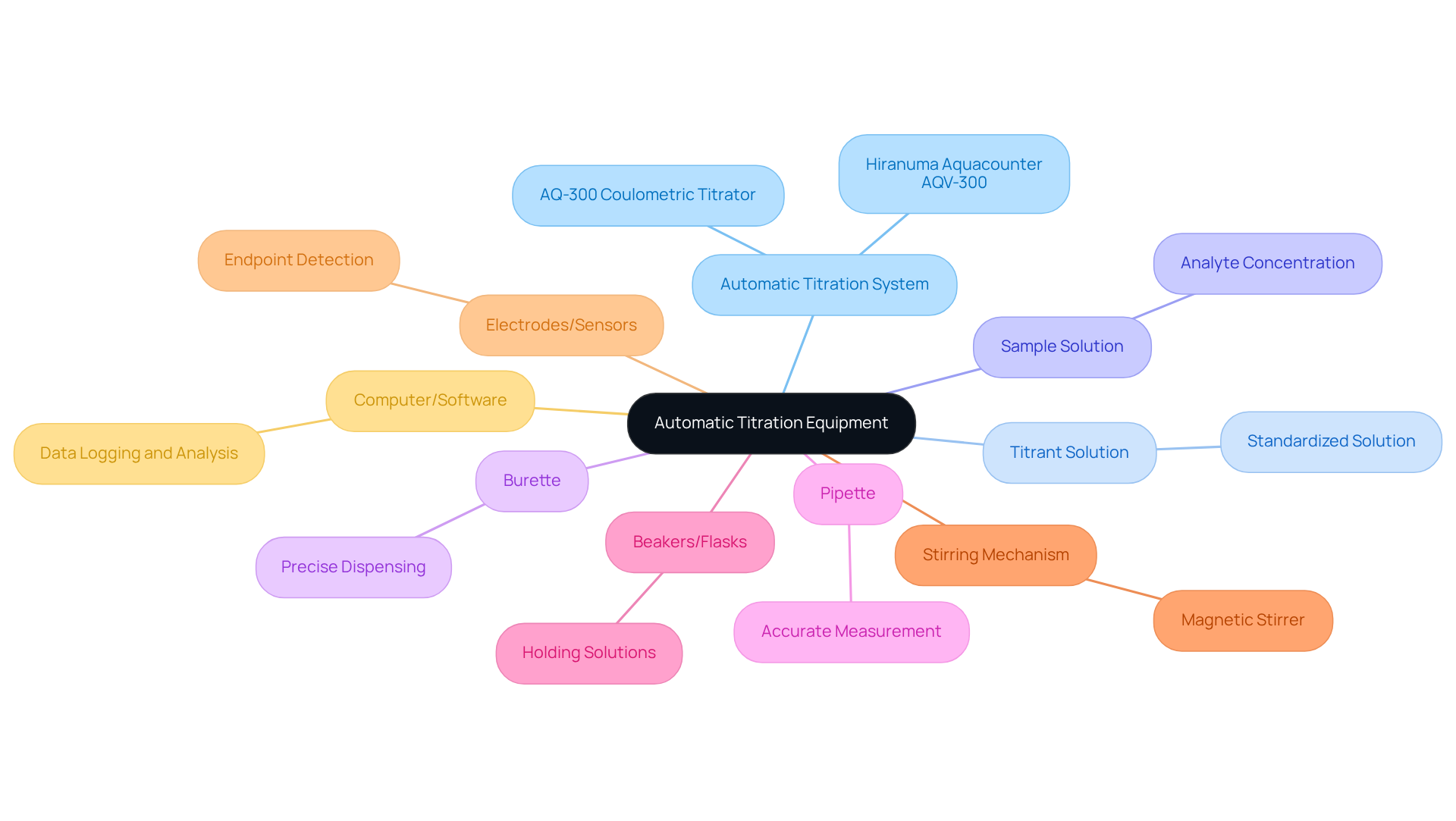
To effectively establish an automatic titration system, certain equipment and materials are indispensable:
- The automatic titration system is a core instrument that automates the titration process, enhancing both precision and efficiency. Notably, the Hiranuma Aquacounter AQV-300 Volumetric Karl Fischer Titrator and AQ-300 Coulometric Karl Fischer Titrator are specifically designed for drug and medicine testing. They ensure adherence to the Japanese Pharmacopoeia, delivering dependable outcomes in pharmaceutical applications.
- Titrant Solution: A standardized solution with a known concentration, this is crucial for reacting with the analyte.
- Sample Solution: This is the solution whose concentration you aim to determine, typically requiring accurate measurement.
- Burette: Employed for the precise dispensing of the titrant, it ensures accurate volume delivery.
- Pipette: Essential for accurately measuring the sample volume, it contributes significantly to the reliability of results.
- Beakers or Flasks: These are essential for holding both the sample and titrant solutions during the measurement process.
- Stirring Mechanism: A magnetic stirrer or similar device is recommended to ensure thorough mixing of the solutions, which is vital for accurate endpoint detection.
- Electrodes or Sensors: Depending on the method used, specific electrodes may be necessary for effective endpoint detection.
- Computer or Software: For data logging and analysis, particularly if your titrator is equipped with advanced connectivity features.
Before initiating the setup, it is crucial to confirm that all devices, including the titrator, are correctly calibrated and in optimal working condition. This step is essential for ensuring precise and dependable results. Notably, volumetric calibration demonstrates a correlation coefficient of 0.9998 between manual and machine volumetric readings, underscoring the significance of accuracy in measurement processes.
Set Up Your Automatic Titration System: A Step-by-Step Process
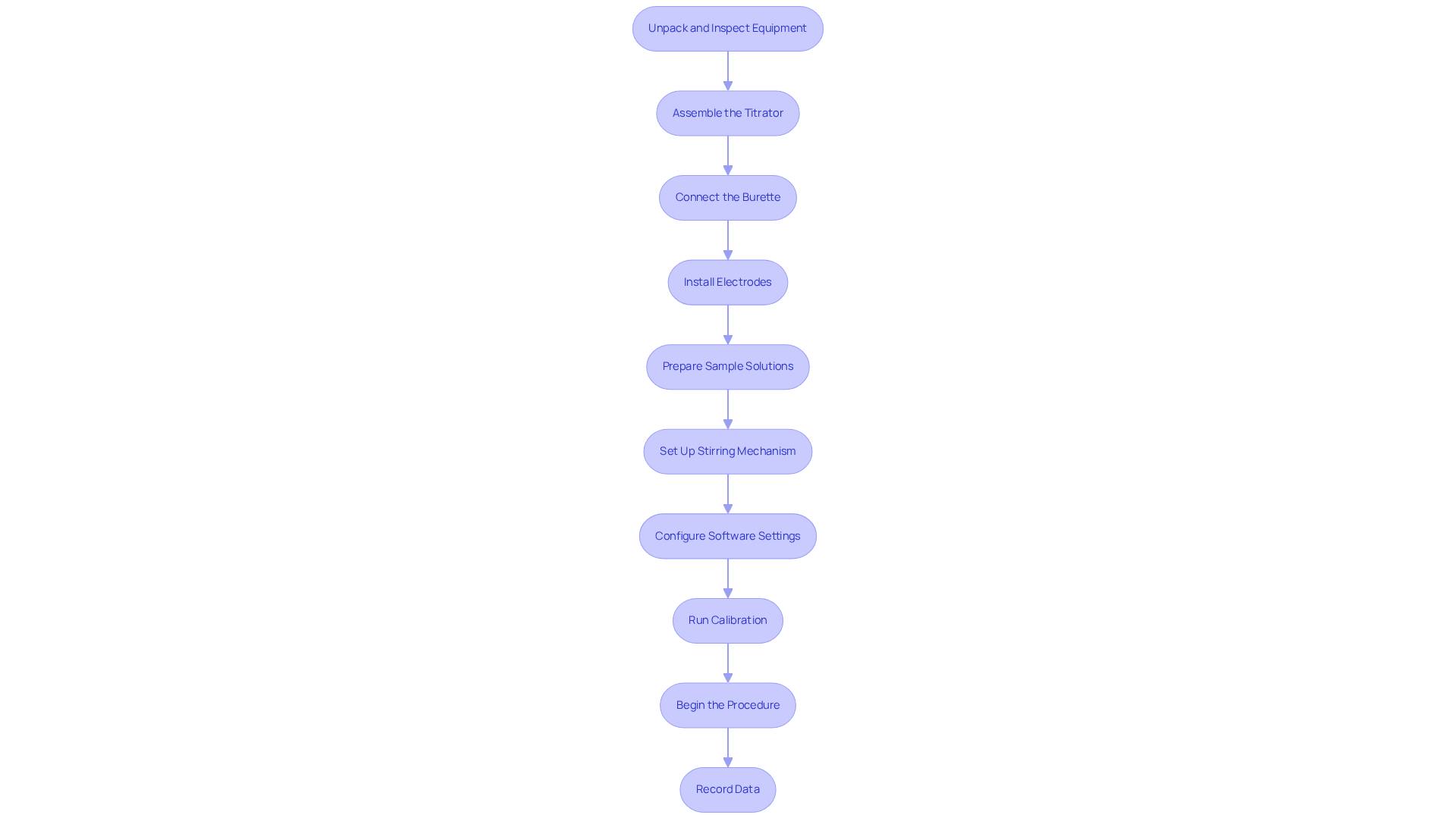
To set up your automatic titration system effectively, follow these essential steps:
-
Unpack and Inspect Equipment: Begin by carefully unpacking all components of the automatic titrator. Inspect each part for any signs of damage, as this is crucial for ensuring optimal performance.
-
Assemble the Titrator: Adhere to the manufacturer's instructions during assembly. It is vital that all parts are securely connected to guarantee the system functions correctly.
-
Connect the Burette: Next, attach the burette to the titrator. Ensure it is filled with the titrant solution and that no air bubbles are present, as these can affect the accuracy of your measurements.
-
Install Electrodes: If applicable, proceed to install the necessary electrodes or sensors for endpoint detection. Proper calibration of these components is essential for reliable results.
-
Prepare Sample Solutions: Measure the required volume of the sample solution using a pipette, and transfer it to a clean beaker or flask. This step is crucial for maintaining the integrity of your analysis.
-
Set Up Stirring Mechanism: Position the stirring mechanism within the sample solution to ensure comprehensive mixing throughout the procedure. This will enhance the accuracy of your titration.
-
Configure Software Settings: If your titrator includes software, adjust the settings according to the type of analysis you are conducting. This includes specifying titrant concentration and the expected endpoint, which are critical for successful titration.
-
Run Calibration: Perform a calibration of the system following the manufacturer's guidelines. This step is vital for ensuring the accuracy of your measurements.
-
Begin the Procedure: Initiate the titration process by pressing the start button. Monitor the progress through the interface, ensuring that everything operates smoothly.
-
Record Data: Finally, ensure that all information is logged accurately. Review the results once the process is complete to verify the success of your titration.
By following these steps, you will effectively establish your automatic titration measurement setup, ensuring optimal performance and accuracy in your laboratory work.
Troubleshoot Common Issues in Automatic Titration Implementation
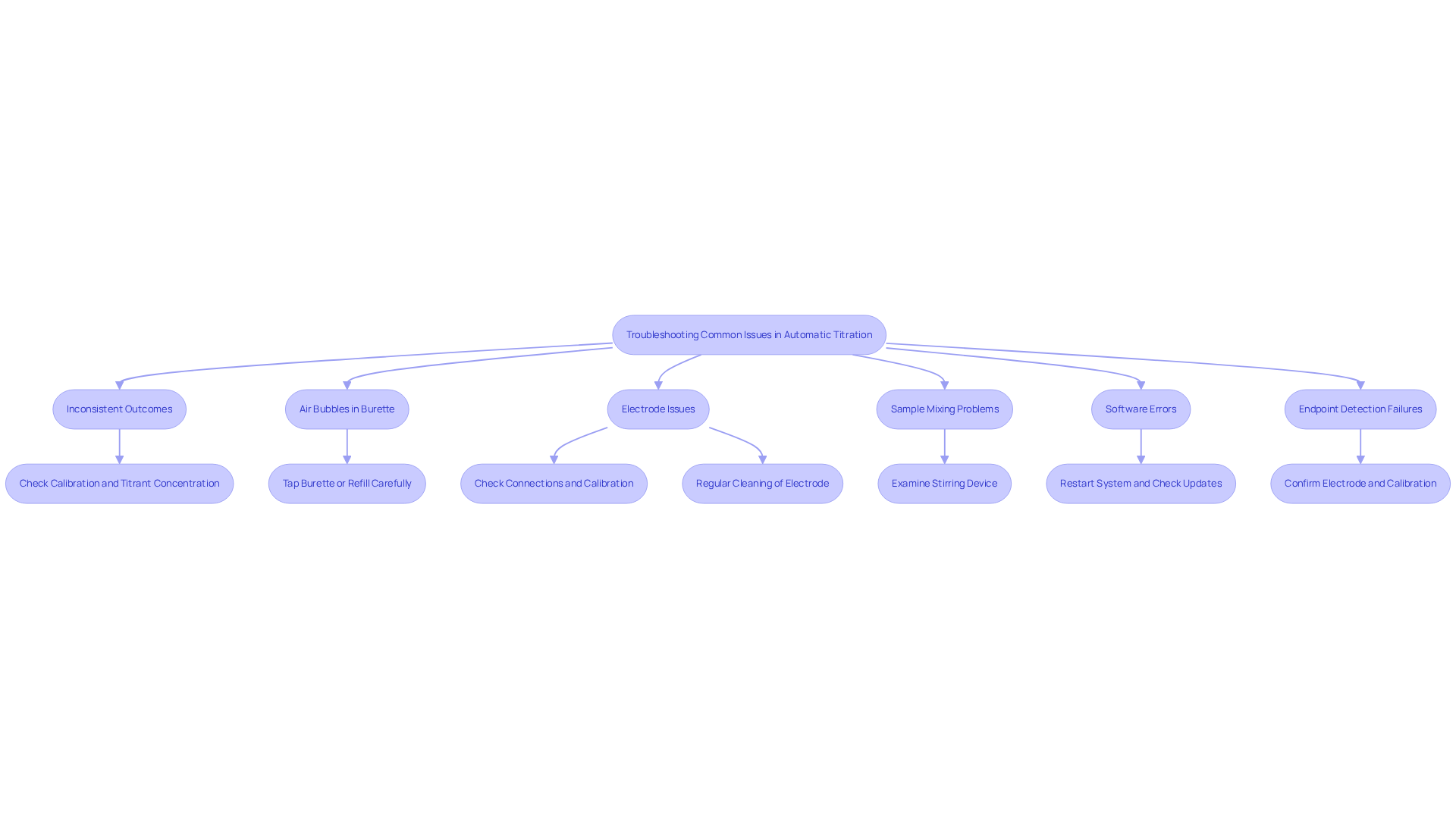
Implementing automatic titration introduces several common challenges that can affect system performance. To enhance your system's efficiency and accuracy, consider the following troubleshooting tips:
-
Inconsistent Outcomes: Variability in results often indicates calibration issues. It is crucial to ensure that the titrator is calibrated correctly and that the titrant concentration is precise. Even minor discrepancies can lead to significant variations in outcomes. For instance, Karl Fischer titration can measure water content from approximately 1 to 50,000 ppm, underscoring the importance of meticulous calibration.
-
Air Bubbles in Burette: The presence of air bubbles can disrupt titrant flow. To eliminate them, gently tap the burette or refill it carefully, ensuring a smooth flow of titrant.
-
Electrode Issues: If the electrode fails to respond, check its connections and calibration. Regular cleaning is essential for maintaining optimal performance. Philippe Lam, a Senior Engineer for Pharmaceutical Process Research and Development, noted that a well-maintained electrode is vital for achieving precise outcomes.
-
Sample Mixing Problems: Inadequate mixing can adversely affect results. Examine the stirring device to ensure it operates correctly, as effective mixing is crucial for accurate measurement.
-
Software Errors: If the software fails to log data or displays errors, restart the system. Ensure that software updates are applied and consult the user manual for troubleshooting guidance.
-
Endpoint Detection Failures: If the endpoint is not identified, confirm that the appropriate electrode is in use and accurately calibrated for the specific analysis method. This step is essential for attaining precise outcomes.
By proactively addressing these challenges, you can significantly improve the efficiency and accuracy of your automatic titration system, ensuring reliable analytical results. Additionally, consider utilizing the HI-931 automatic titration system, renowned for its versatility and capability to perform a variety of standard and custom potentiometric titrations, further enhancing your laboratory's analytical capabilities.
Conclusion
In conclusion, automatic titration signifies a monumental leap forward in laboratory analysis, optimizing the determination of solution concentrations with unmatched precision and efficiency. By automating the titration process, laboratories can significantly reduce human error, bolster safety measures, and enhance data management, ultimately yielding more dependable and consistent results.
Throughout this discourse, we have explored the fundamental principles and advantages of automatic titration, outlined the essential equipment required for effective implementation, and provided a thorough step-by-step guide for establishing a robust system. Moreover, we addressed common challenges linked to automatic titration, offering practical troubleshooting strategies to maximize system performance. Each of these components deepens our comprehension of how automatic titration can revolutionize laboratory workflows.
Adopting automatic titration not only improves the quality of analytical outcomes but also cultivates a safer, more efficient laboratory atmosphere. By incorporating this innovative technology, laboratories can elevate their operational capabilities and ensure adherence to industry standards. Transitioning to automatic titration transcends a mere technical upgrade; it represents a strategic initiative toward achieving excellence in analytical chemistry.




