Overview
This article delves into essential techniques that lab managers must master regarding chromatography plates. Understanding the interaction between stationary and mobile phases is paramount for the effective separation of mixtures. To support this, the article details best practices, troubleshooting common issues, and discusses various types of chromatography plates. These elements are crucial for enhancing analytical outcomes in laboratory settings, underscoring the importance of high-quality scientific instruments. By mastering these techniques, lab managers can significantly improve their laboratory's performance.
Introduction
Chromatography plates are indispensable tools in analytical chemistry, playing a crucial role in the separation of complex mixtures into their individual components. Their significance extends beyond mere functionality; understanding the intricacies of these plates enhances laboratory efficiency and ensures precise, reliable results across various applications—from pharmaceuticals to environmental testing.
However, the complexity of selecting the right type of plate and optimizing its use poses a challenge. How can lab managers navigate these hurdles to improve their analytical outcomes? This question not only invites deeper exploration but also underscores the necessity for informed decision-making in laboratory settings.
Explore the Fundamentals of Chromatography Plates
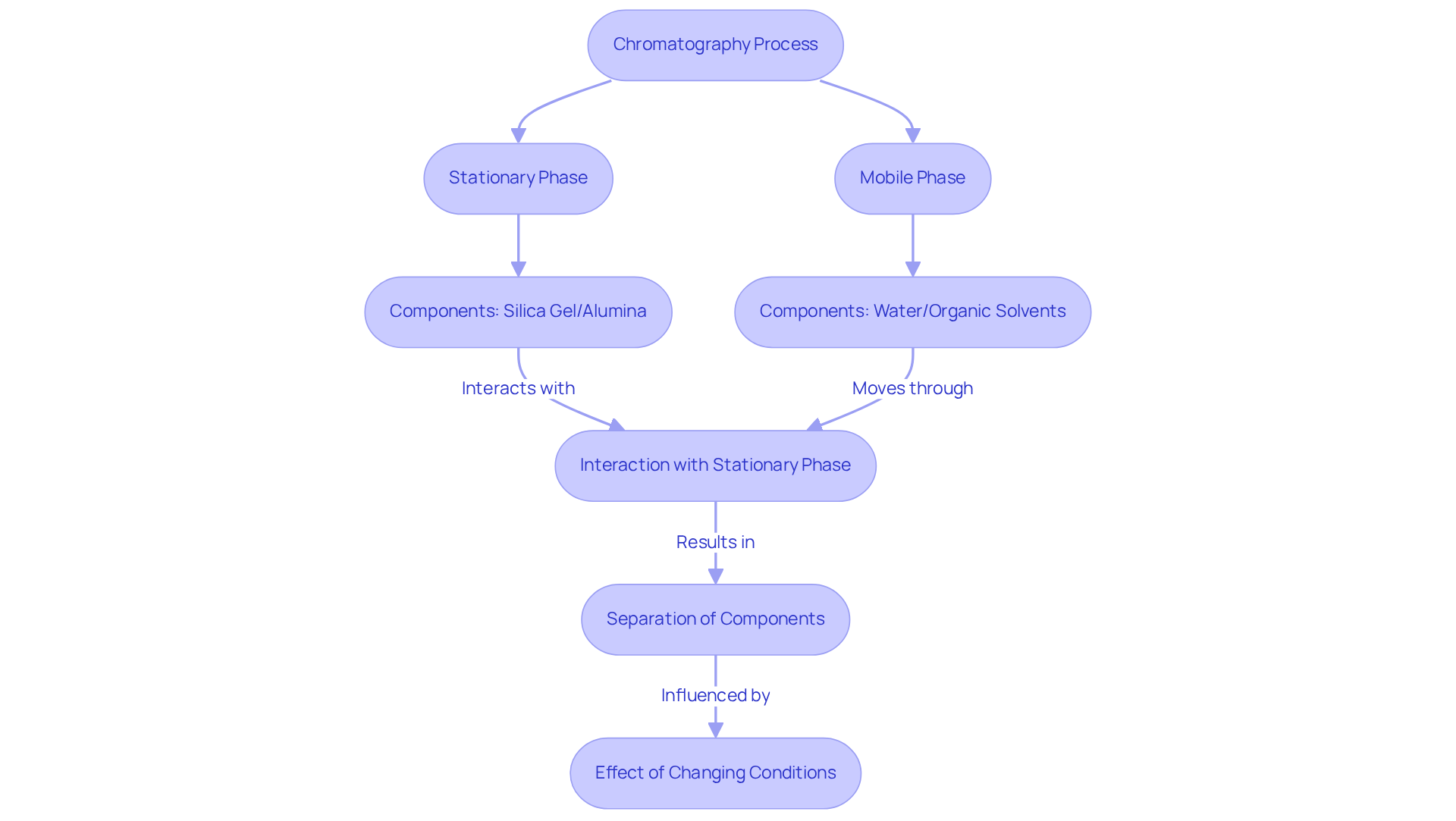
Chromatography plates serve as essential tools in analytical chemistry, facilitating the separation of mixtures into distinct components. The fundamental principle operates in two stages: the stationary stage, typically composed of silica gel or alumina, and the mobile stage, which is the solvent traversing the stationary phase. As the mobile segment ascends along the surface, the various components of the mixture interact with the stationary medium to differing extents, resulting in their separation. This interaction is pivotal for enhancing chromatography plates used in laboratory environments.
The composition of the stationary phase significantly impacts separation efficiency. For instance, employing hydrocarbon-bonded silica gels, such as C18 and C8 columns, has demonstrated improved selectivity and reproducibility in liquid chromatography. Statistical analyses indicate that the retention factor (Rf), a critical metric representing the distance a compound moves relative to the fluid front, ideally ranges between 0.2 and 0.4 for optimal separation. This range ensures adequate resolution of compounds without excessive overlap.
In practical applications, selecting the mobile medium is equally crucial. Mobile mixtures typically consist of combinations of water and organic solvents, such as methanol or acetonitrile, which can be adjusted to enhance separation outcomes. For example, research has shown that altering the mobile phase composition can significantly influence the retention of nitrobenzenes, with their Rf values remaining consistent across various conditions, underscoring the method's robustness.
The real-world applications of chromatography plates span multiple fields, including pharmaceuticals, environmental testing, and food safety. In pharmaceutical laboratories, for example, separation techniques are employed to assess drug purity and stability, ensuring compliance with regulatory standards. The ability to modify stationary and mobile components allows chemists to tailor their techniques for specific analytes, enhancing both precision and effectiveness in analytical procedures.
Understanding the interaction between stationary and mobile phases is essential for lab managers aiming to improve separation techniques. By leveraging insights from analytical chemists and statistical data, laboratories can bolster their analytical capabilities, resulting in more reliable and reproducible outcomes. Furthermore, the global market for analytical tools and consumables was valued at approximately US$4.79 billion in 2023 and is projected to grow at a CAGR of 7.5% from 2025 to 2030, emphasizing the significance of this technique within the sector. Advancements such as automated high-throughput TLC platforms that measure R values for various compounds exemplify the ongoing evolution in chromatography technology.
Identify Different Types of Chromatography Plates and Their Applications
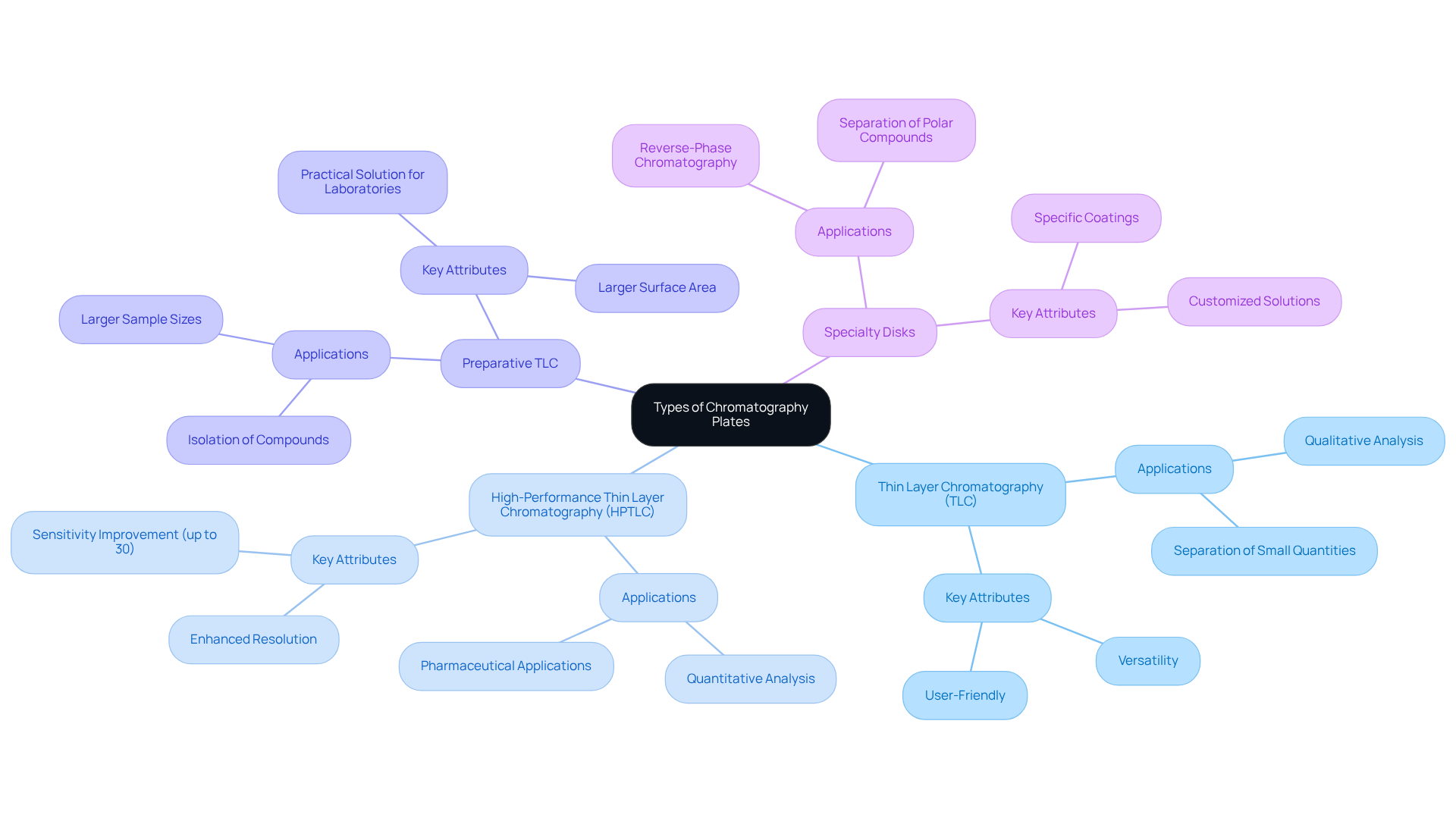
Available in several types, chromatography plates are specifically designed for distinct applications, capturing the attention of laboratory professionals.
Thin Layer Chromatography (TLC) Surfaces stand out for their versatility and user-friendliness, making them widely utilized for qualitative analysis. They excel in separating small quantities of compounds, establishing their role as a staple in many laboratories. The market for chromatography plates is projected to reach USD 29.5 billion by 2031, underscoring their significance in the industry.
High-Performance Thin Layer Chromatography (HPTLC) Surfaces provide enhanced resolution and sensitivity, making them ideal for quantitative analysis in complex mixtures. With a projected rapid Compound Annual Growth Rate (CAGR) during the forecast period, HPTLC's growing importance in pharmaceutical applications is evident, particularly due to its ability to deliver precise results quickly. Studies reveal that HPTLC can enhance sensitivity by up to 30% compared to traditional TLC methods, reinforcing its value.
Preparative TLC Surfaces are tailored for larger sample sizes, proving essential when significant amounts of material need separation and collection. Their larger surface area facilitates the isolation of compounds for further analysis, offering laboratories a practical solution for their needs.
Specialty Disks encompass disks with specific coatings or modifications designed for unique applications, such as reverse-phase chromatography, critical for separating polar compounds. The creation of specialized discs is increasingly significant as laboratories seek customized solutions for particular analytical challenges.
Understanding the specific uses of each category of chromatography plates is crucial for laboratory supervisors. Selecting the appropriate chromatography plates not only enhances experimental results but also aligns with the laboratory's analytical objectives. As sustainability becomes increasingly important in the TLC surface market, lab managers are encouraged to explore eco-friendly alternatives that meet their analytical requirements.
Implement Best Practices for Using Chromatography Plates in the Lab
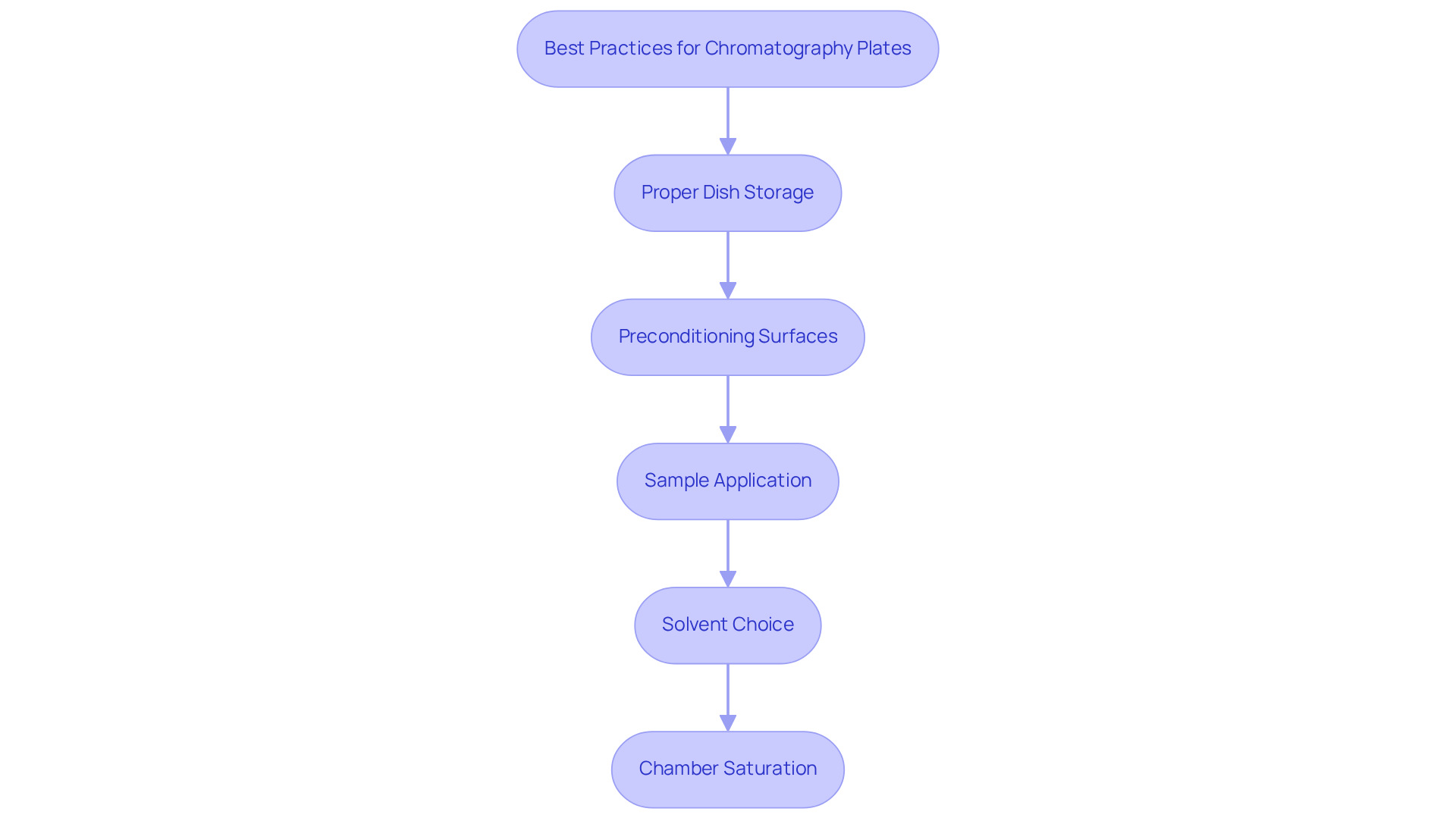
To achieve optimal results with chromatography plates, it is essential to adhere to the following best practices:
-
Proper Dish Storage: Store dishes in a clean, dry environment to prevent contamination. Wrapping them in aluminum foil offers protection against moisture and chemical vapors, thereby ensuring longevity and reliability.
-
Preconditioning Surfaces: Prior to use, precondition surfaces by heating them to eliminate any bound moisture. This step is crucial, as moisture can significantly interfere with chromatographic results, adversely affecting separation quality.
-
Sample Application: Employ a capillary tube for sample application to guarantee precise and consistent spotting. It is imperative to avoid overloading the plate, as excessive sample amounts can lead to poor separation and compromised results.
-
Solvent Choice: Select an appropriate solvent system based on the polarity of the compounds being analyzed. For acidic compounds, consider incorporating acetic or formic acid at concentrations ranging from 0.1% to 2.0%. Common mixtures, such as a 1:1 ratio of ethyl acetate to hexanes, may serve as initial starting points; however, optimizing the ratios of the liquids may necessitate some experimentation.
-
Chamber Saturation: Ensure that the developing chamber is saturated with vapor before placing the plate inside. The liquid level within the TLC chamber should be approximately 0.5 cm. This practice is vital for maintaining consistent liquid front movement, which is essential for reproducible results.
Implementing these methods not only enhances the effectiveness of chromatography plates but also results in more precise and dependable analytical outcomes. A case study on improving solvent systems for column separation underscores the importance of assessing solvent conditions on TLC surfaces to achieve satisfactory separations.
Troubleshoot Common Issues with Chromatography Plates
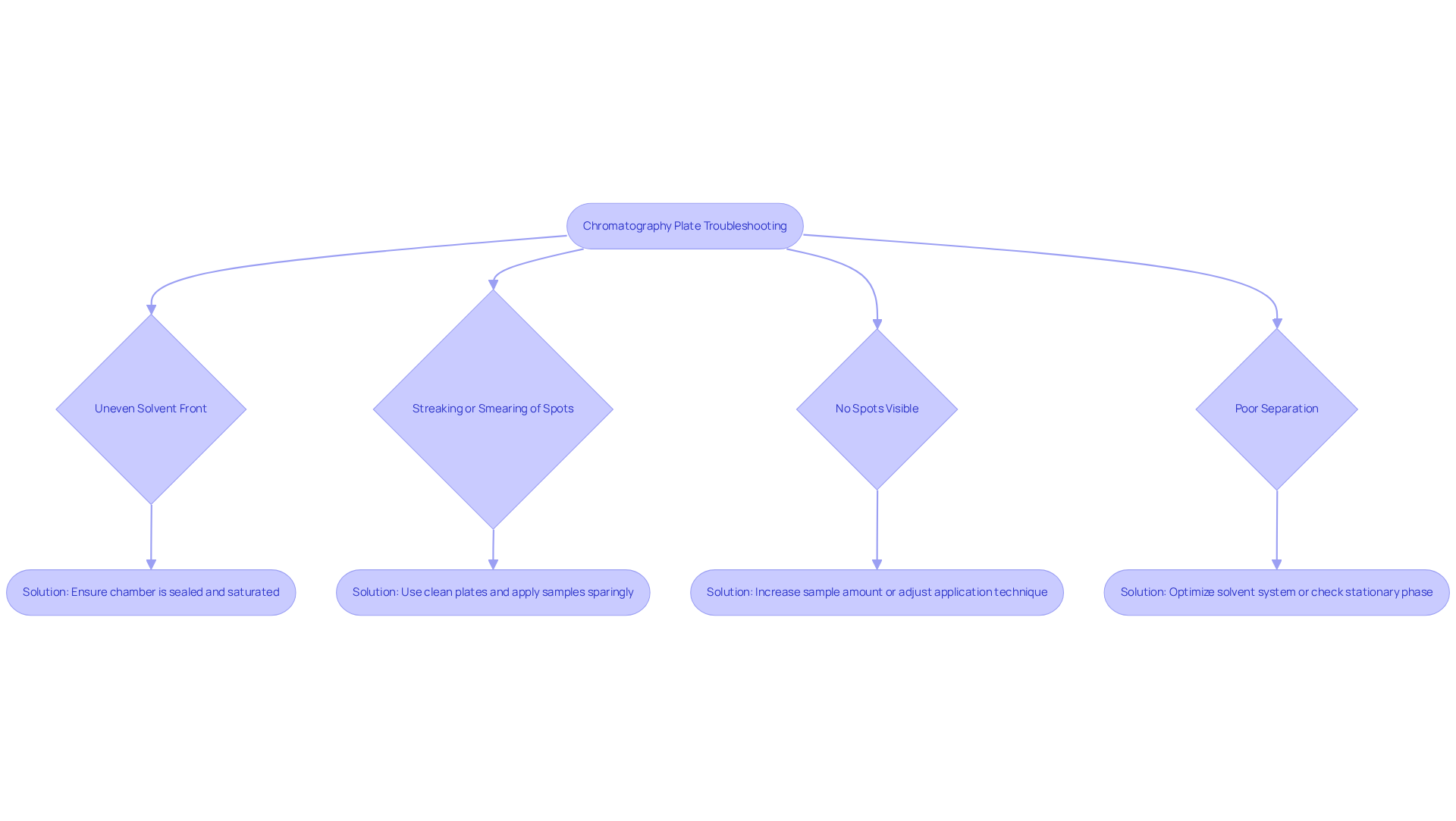
Common issues encountered when utilizing chromatography plates involve several key challenges that can impact the outcome of your experiments.
-
An uneven solvent front may arise if the chamber is not properly saturated. It is essential to ensure that the chamber is adequately sealed and to allow sufficient time for saturation before use.
-
Streaking or smearing of spots can occur, often as a result of applying too much sample or working with a contaminated surface. To mitigate this, always utilize clean plates and apply samples sparingly to achieve optimal results.
-
If no spots are visible after development, this may indicate that the sample concentration is too low. In such cases, consider increasing the sample amount or adjusting your application technique for better visibility.
-
Poor separation can be identified if bands appear broad or poorly defined. It is advisable to optimize the solvent system or check for potential issues with the stationary phase to enhance separation quality.
By recognizing these common problems and their respective solutions, lab managers can uphold high standards of quality and efficiency in their chromatography work, ultimately leading to more reliable and reproducible results.
Conclusion
Mastering chromatography plates is essential for laboratory managers aiming to enhance analytical precision and efficiency. These plates transcend their role as mere tools; they encapsulate the intricate science of separating complex mixtures into identifiable components, a fundamental process across various scientific domains, including pharmaceuticals and environmental testing.
This article explores the critical aspects of chromatography plates, addressing the principles of operation, the types available, best practices for usage, and common troubleshooting techniques. It underscores the importance of understanding both the stationary and mobile phases, selecting the appropriate plate type for specific applications, and implementing best practices to achieve optimal results. Furthermore, recognizing and addressing common issues can significantly bolster the reliability and reproducibility of analytical outcomes.
In summary, the effective utilization of chromatography plates is paramount for laboratory success. By adopting the insights and best practices outlined here, lab managers can not only enhance the quality of their analytical results but also contribute to the ongoing advancement of laboratory techniques. Embracing innovation and staying informed about developments in chromatography technology will further empower laboratories to meet the evolving demands of the scientific community.




