Overview
This article delineates the essential steps for successful chromatography sample preparation, underscoring the critical role of meticulous techniques in achieving accurate analytical results. It presents key procedures and necessary tools, while also addressing common troubleshooting strategies. Proper sample preparation is not merely a procedural formality; it is vital for ensuring the precision and reproducibility of chromatography analyses. By adhering to these guidelines, practitioners can significantly enhance the reliability of their results, fostering confidence in their analytical capabilities.
Introduction
Chromatography stands as a pivotal analytical technique, essential for the separation and analysis of complex mixtures, especially within the realms of chemistry and pharmaceuticals. Mastering the intricacies of chromatography sample preparation is crucial, as it directly impacts the accuracy and reliability of analytical results.
Nevertheless, many professionals encounter challenges that hinder optimal outcomes, often stemming from common pitfalls in the preparation process.
To enhance sample preparation and ensure precision in chromatography, what strategies can be employed?
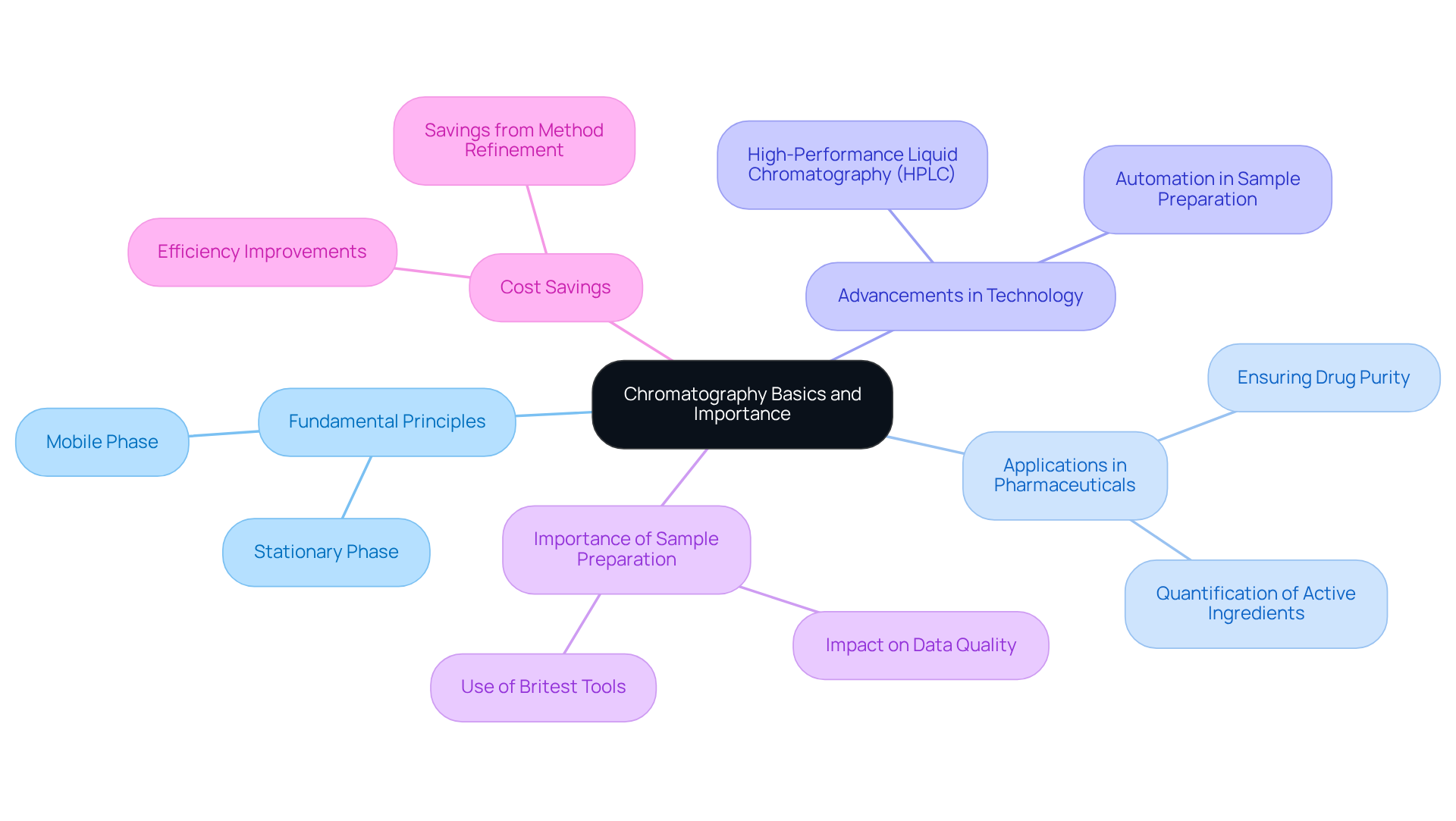
Understand Chromatography Basics and Importance
Chromatography stands as a cornerstone analytical technique, essential for the separation and analysis of components within complex mixtures, with significant applications in chemistry, biochemistry, and pharmaceuticals. Understanding the fundamental principles of chromatography—specifically the roles of stationary and mobile phases—is crucial for effective utilization. The stationary phase remains unchanged, while the mobile phase, a solvent, traverses through the stationary phase, carrying the specimen. The effectiveness of separation hinges on factors such as the selection of stationary phase materials, the composition of the mobile phase, and the characteristics of the substance being analyzed.
In the pharmaceutical sector, separation techniques are vital for ensuring the purity and efficacy of drug formulations. Recent advancements in high-performance liquid separation techniques have revolutionized the analysis of complex biological samples, enabling precise quantification of active pharmaceutical ingredients. JM Science Inc. offers a range of premium HPLC columns and accessories, including Shodex and CapcellPak columns, specifically designed to enhance chromatographic efficiency and accuracy. Furthermore, the company provides titrators and Karl Fischer reagents, further supporting analytical needs in the pharmaceutical industry. Industry leaders emphasize the necessity of mastering chromatography fundamentals to optimize analytical techniques and achieve dependable outcomes. Analytical scientists at AstraZeneca have noted that refining test methods can yield substantial cost savings, estimated between $10,000 and $50,000 for each out-of-specification occurrence prevented.
Moreover, meticulous chromatography sample preparation is critical, as it directly influences the precision and reproducibility of analytical outcomes. Effective chromatography sample preparation techniques significantly enhance data quality, thereby facilitating informed decision-making in research and development. The utilization of Britest tools to visualize the preparation process and identify sources of variation underscores the importance of this step. By comprehending and employing separation techniques, professionals can markedly advance analytical chemistry and the pharmaceutical industry, bolstered by the innovative solutions provided by JM Science.
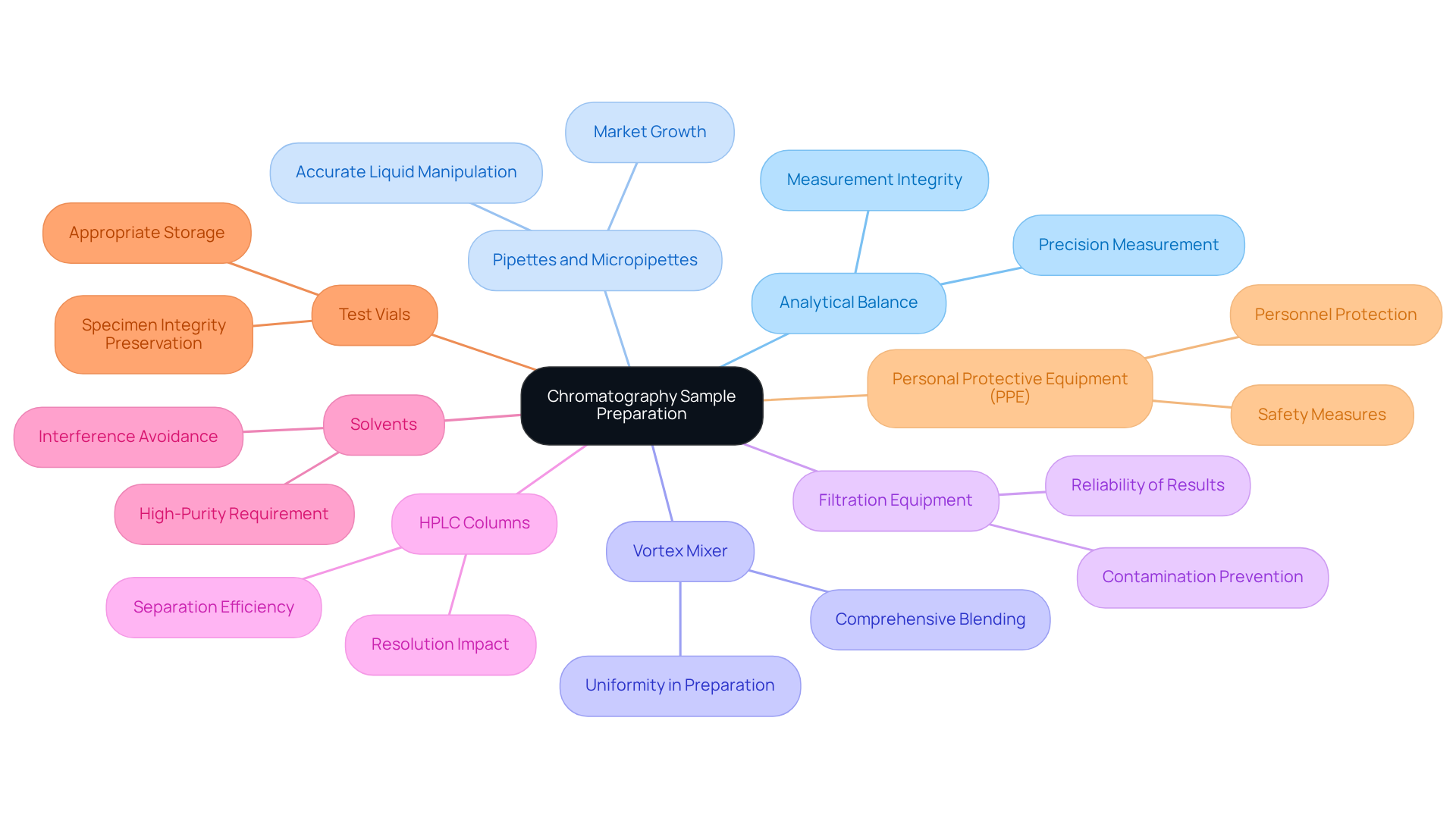
Gather Essential Tools and Materials for Sample Preparation
Several essential tools and materials are required to effectively prepare samples for chromatography sample preparation.
-
Analytical Balance: This instrument is crucial for the precise measurement of sample quantities, ensuring accuracy in your analyses. Laboratory managers emphasize that high-quality analytical balances are vital for maintaining measurement integrity, as even minor discrepancies can lead to significant errors in results. According to PerkinElmer, the simplification of specimen preparation processes has greatly enhanced laboratory efficiency.
-
Pipettes and Micropipettes: These instruments enable accurate liquid manipulation, allowing for the precise transfer of reagents and materials. The market for pipettes, particularly micropipettes, has shown robust growth, with non-robotic pipette tips holding a significant revenue share of 56.4% in 2021, indicating their continued importance in laboratory settings.
-
Vortex Mixer: A vortex mixer guarantees comprehensive blending of materials, which is crucial for achieving uniformity in preparation.
-
Filtration Equipment: Utilizing syringe filters helps remove particulates from samples, preventing contamination and ensuring the reliability of chromatographic results.
-
HPLC Columns: Choosing the suitable HPLC columns is essential, as they are specific to the type of separation being conducted, affecting separation efficiency and resolution.
-
Solvents: High-purity solvents compatible with your chromatography method are necessary to avoid interference and ensure optimal performance.
-
Test Vials: Proper storage of prepared specimens in appropriate vials is crucial for preserving specimen integrity before examination.
-
Personal Protective Equipment (PPE): Safety must never be disregarded; gloves and goggles are essential to safeguard personnel during specimen preparation.
Collecting these materials beforehand will simplify the chromatography sample preparation process and help maintain a tidy and structured workspace, ultimately improving the efficiency and precision of your analyses.
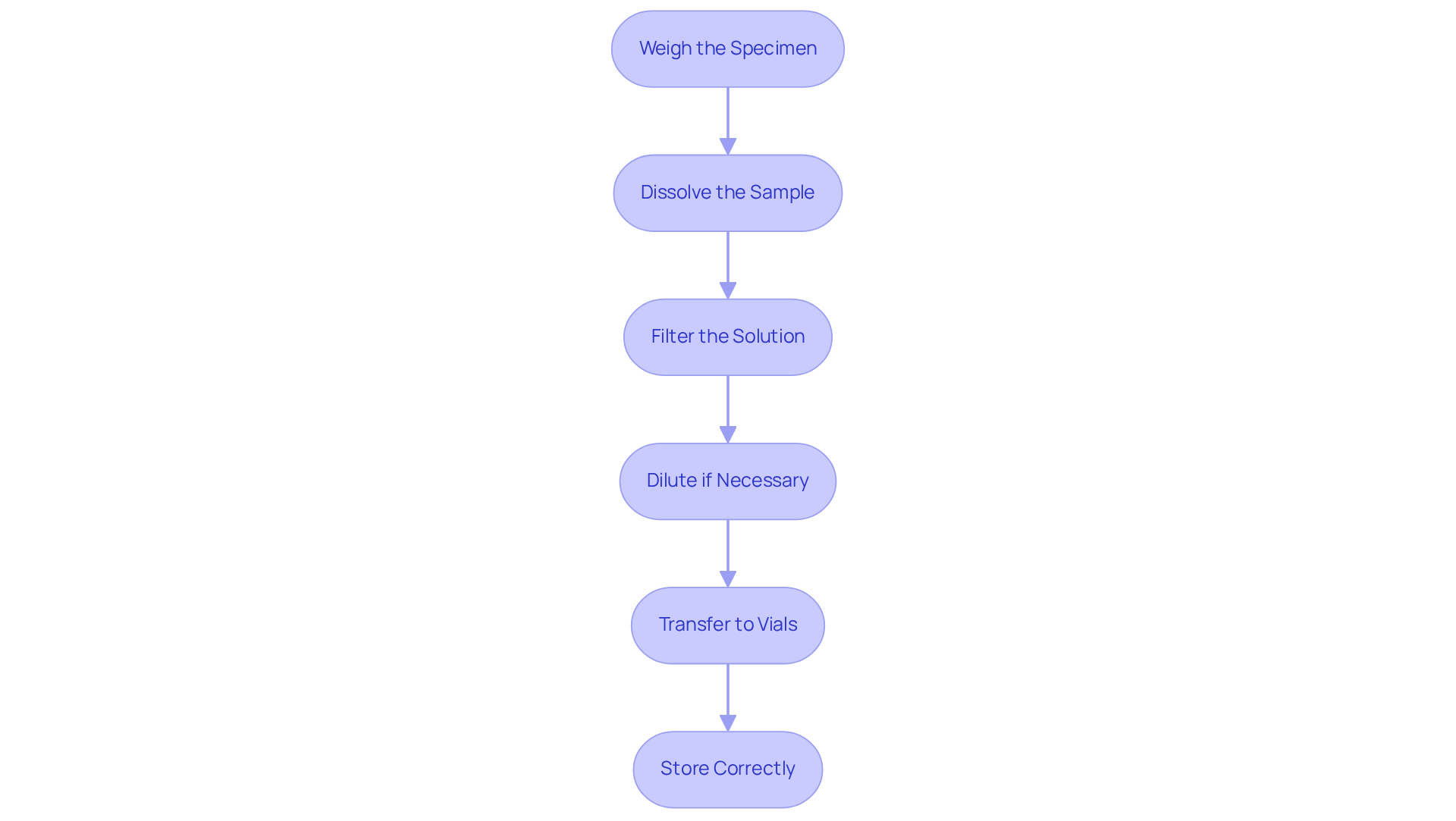
Follow Step-by-Step Sample Preparation Procedures
-
Weigh the Specimen: Begin by utilizing an analytical balance to measure the precise quantity of your specimen. It is imperative that the balance is calibrated to ensure optimal accuracy. Accurate weighing is critical in chromatography sample preparation, as even minor discrepancies can significantly impact the results. As Dr. Sujatha Mahadevarao Premnath emphasizes, 'Regular calibration of instruments, appropriate handling of materials, and meticulous record-keeping are essential to maintain precision.'
-
Dissolve the Sample: Next, transfer the weighed sample into a clean container and introduce an appropriate solvent. Employ a vortex mixer to ensure thorough dissolution, as incomplete dissolution can lead to inconsistent outcomes, adversely affecting peak resolution in HPLC analysis. Research indicates that the technique of dissolution can influence overall findings, underscoring the importance of this stage.
-
Filter the Solution: Subsequently, pass the dissolved substance through a syringe filter to remove any particulates that could interfere with the chromatography process. This step is crucial for maintaining the integrity of the chromatographic system in chromatography sample preparation and ensuring reliable data.
-
Dilute if Necessary: Depending on the concentration of your specimen, it may be necessary to dilute with the mobile phase solvent to achieve optimal results. Proper dilution can enhance the sensitivity and precision of the examination, particularly in complex matrices.
-
Transfer to Vials: Carefully transfer the prepared material into clean vials, ensuring they are accurately labeled. Precise labeling is vital for traceability and helps avoid mix-ups during examination.
-
Store Correctly: If immediate examination is not feasible, store the specimens under suitable conditions to prevent deterioration. Appropriate storage is essential for preserving integrity and ensuring trustworthy outcomes.
By adhering to these meticulous procedures, you will ensure that your specimens undergo effective chromatography sample preparation for evaluation, ultimately resulting in more precise and reproducible outcomes.
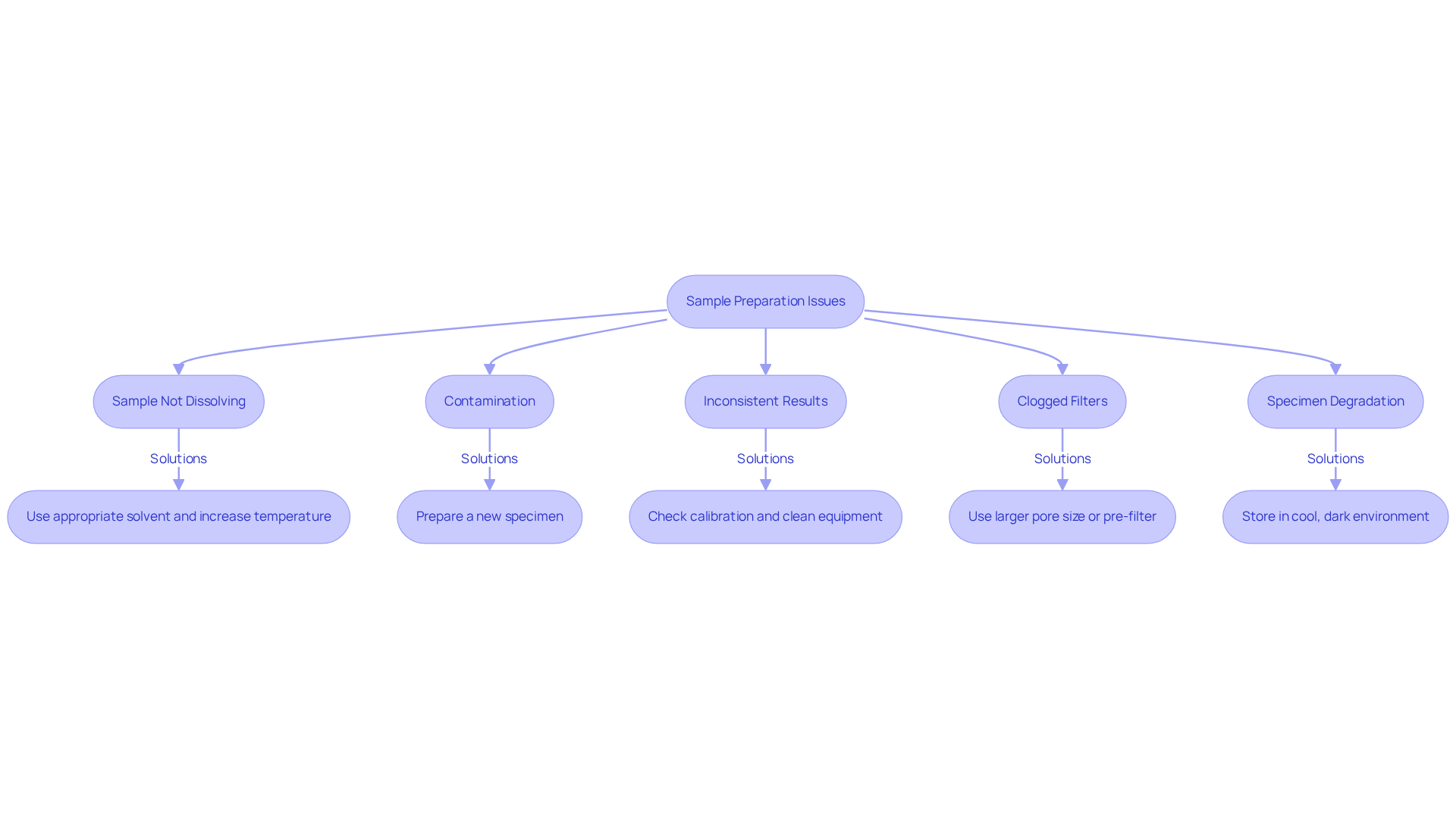
Troubleshoot Common Sample Preparation Issues
-
Sample Not Dissolving: If your sample fails to dissolve completely, it is essential to verify that you are utilizing the appropriate solvent. Slightly increasing the temperature or employing a vortex mixer can significantly enhance mixing efficiency. Research has shown that optimizing solvent choice and conditions can lead to improved dissolution rates, which is crucial for accurate analysis. Addressing such issues can prevent out-of-specification results, potentially saving between $10,000 and $50,000 per incident.
-
Contamination: To prevent contamination, always utilize clean equipment and avoid direct contact with the interior surfaces of vials or filters. If contamination is suspected, it is advisable to prepare a new specimen. Laboratory experts emphasize that preserving specimen integrity is crucial; even slight contamination can result in considerable analytical mistakes. Expert quotes underscore the importance of rigorous contamination prevention measures.
-
Inconsistent Results: When faced with significant variability in results, it is vital to check the calibration of your analytical balance and ensure that all equipment is clean and functioning correctly. A structured review of manufacturing operations has demonstrated that addressing calibration issues can enhance measurement precision, thereby improving overall data reliability. For instance, Biogen's evaluation recognized important sources of variation in specimen preparation that, when resolved, resulted in enhanced outcomes.
-
Clogged Filters: If filters become blocked, consider using a larger pore size or pre-filtering the material to eliminate larger particulates. Implementing these strategies can prevent bottlenecks in the analytical process, as evidenced by case studies where filter optimization led to increased throughput and reduced downtime. A Britest study revealed that addressing long-standing pressure filtration issues saved a large manufacturing organization £500K annually.
-
Specimen Degradation: To mitigate specimen degradation, keep specimens in a cool, dark environment and examine them promptly. Research emphasizes that suitable storage conditions are crucial for maintaining sample integrity, which directly affects the precision of chromatography sample preparation and outcomes. Highlighting the significance of these conditions can greatly improve the reliability of your analytical results.
By proactively addressing these common issues and implementing the suggested solutions, you can significantly enhance the reliability and accuracy of your chromatography sample preparation results.
Conclusion
Mastering chromatography sample preparation is essential for achieving reliable and accurate analytical results. This article underscores the significance of understanding chromatography fundamentals, gathering essential tools, and adhering to meticulous preparation procedures to enhance data quality. By focusing on these critical aspects, professionals in analytical chemistry and pharmaceuticals can optimize their methodologies and ensure the integrity of their results.
Key insights include:
- The necessity of precise measurement with analytical balances
- The importance of using appropriate solvents for dissolution
- The critical role of filtration in preventing contamination
Furthermore, addressing common sample preparation challenges, such as inconsistent results and specimen degradation, highlights the need for vigilance and strict adherence to best laboratory practices.
Ultimately, mastering chromatography sample preparation not only improves analytical outcomes but also fosters advancement in research and development across various fields. By prioritizing these essential steps and employing innovative solutions, professionals can significantly enhance their analytical capabilities, paving the way for more informed decision-making and improved product quality in the pharmaceutical industry.




