Overview
This article provides a comprehensive overview of the effective use of Copper(I) iodide (CuI) in organic synthesis, emphasizing its role as a versatile catalyst in critical processes such as cross-coupling and oxidative coupling.
The discussion begins with an exploration of CuI's properties and preparation methods, establishing its significance in the field.
By detailing troubleshooting strategies, the article demonstrates that CuI not only enhances reaction efficiency but also serves as a cost-effective alternative to traditional catalysts.
This makes it an invaluable asset in the development of complex organic compounds, thereby underscoring its importance in advancing organic synthesis.
Introduction
Copper(I) iodide (CuI) is a remarkable compound in the realm of organic synthesis, distinguished by its unique properties and versatile applications. As chemists strive for efficient and cost-effective catalysts, CuI presents itself as a compelling alternative to traditional options such as palladium or nickel. This is particularly evident in essential cross-coupling reactions that form carbon-carbon bonds.
However, mastering its use involves navigating several challenges:
- Ensuring reagent purity
- Managing stability under varying conditions
The effective application of copper(I) iodide necessitates a nuanced understanding. What strategies can be employed to navigate these complexities and optimize the benefits of this powerful catalyst?
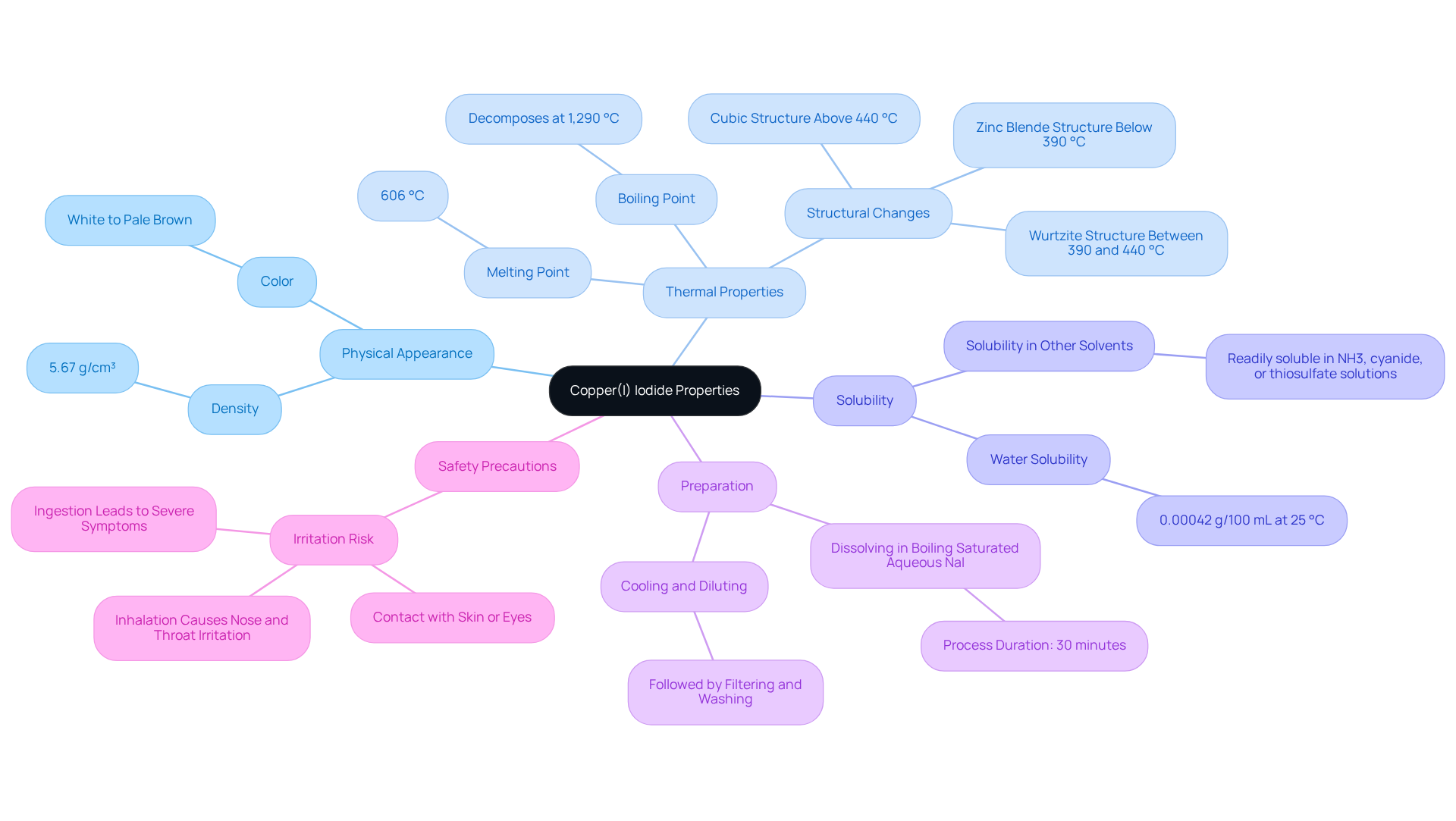
Understand the Properties of Copper(I) Iodide
(CuI) is an inorganic compound notable for its white to pale brown appearance and a density of 5.67 g/cm³. With a melting point of 606 °C and a boiling point of 1,290 °C—where copper 1 iodide decomposes rather than boiling—copper 1 iodide presents unique thermal properties. The low solubility of copper 1 iodide in water, measured at merely 0.00042 g/100 mL at 25 °C, is a critical factor in its application in organic synthesis, as it influences interactions with other reagents.
Copper 1 iodide can be freshly prepared by dissolving it in boiling saturated aqueous NaI over 30 minutes, a process essential for its various applications. Additionally, above 440 °C, copper 1 iodide adopts a cubic structure, which is vital for understanding its stability and behavior under varying conditions.
Copper 1 iodide functions as a reducing agent and is sensitive to oxidation, factors that can significantly influence its stability in diverse environments. It is imperative to recognize that contact with skin or eyes can lead to irritation, underscoring the necessity for safety precautions in laboratory settings. Understanding these properties is crucial for enhancing the application of copper 1 iodide in chemical processes, ensuring both efficacy and safety in its use.
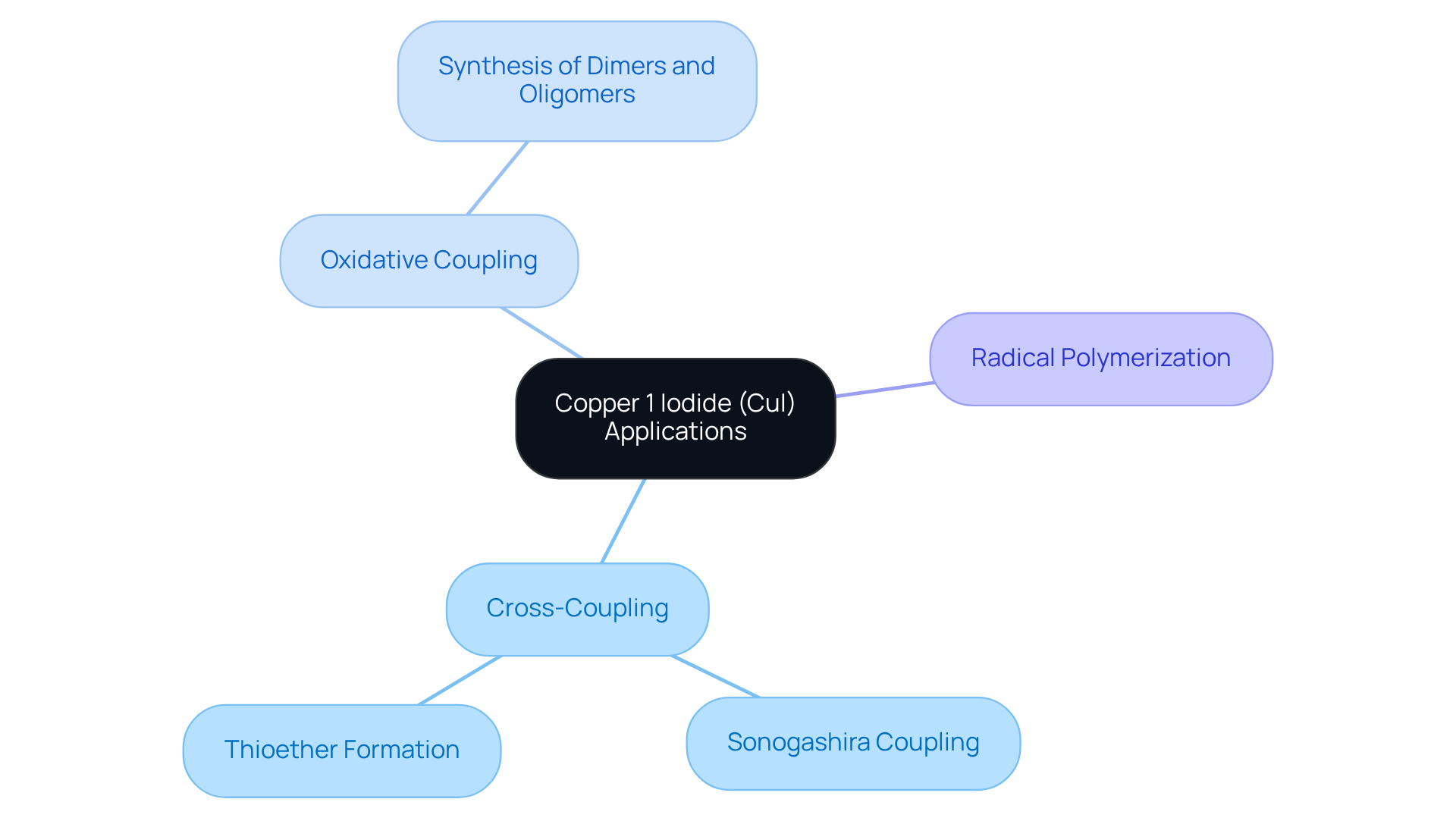
Explore Applications in Organic Synthesis
Copper 1 iodide (CuI) serves as a pivotal [catalyst in organic synthesis](https://jmscience.com), particularly renowned for its role in cross-coupling processes that are vital for the formation of carbon-carbon bonds. One of the most significant applications of copper 1 iodide is in , where it adeptly facilitates the interaction between aryl halides and terminal alkynes, leading to the production of valuable conjugated enynes. Furthermore, copper 1 iodide demonstrates its versatility by catalyzing the formation of thioethers from aryl halides and thiols, underscoring its broad utility in organic transformations.
Recent advancements highlight the efficacy of copper 1 iodide in oxidative coupling processes, where it promotes the joining of similar organic molecules, resulting in the synthesis of dimers or larger oligomers. This capability proves particularly advantageous in the creation of complex organic compounds and materials. Moreover, the ability of copper 1 iodide to mediate radical polymerizations enhances its utility, enabling controlled polymer architecture that is essential for developing innovative materials.
Expert insights further illuminate the significance of CuI in contemporary organic synthesis. As Shihua Wang from the Department of Chemistry at Huaibei Normal University notes, "This method offers a simple, efficient, and cost-effective approach to synthesizing unsymmetrical buta-1,3-diynes, which are valuable in organic synthesis, pharmaceuticals, and materials science." The low cost and reduced toxicity of copper 1 iodide in comparison to traditional catalysts such as palladium or nickel render it an appealing alternative. Additionally, copper 1 iodide has demonstrated impressive product yields of up to 96% in S-Arylation reactions, highlighting its efficiency as a catalyst. As research continues to advance, a deeper understanding of CuI's diverse applications will empower chemists to refine their synthetic strategies, fostering innovation in pharmaceuticals and materials science.
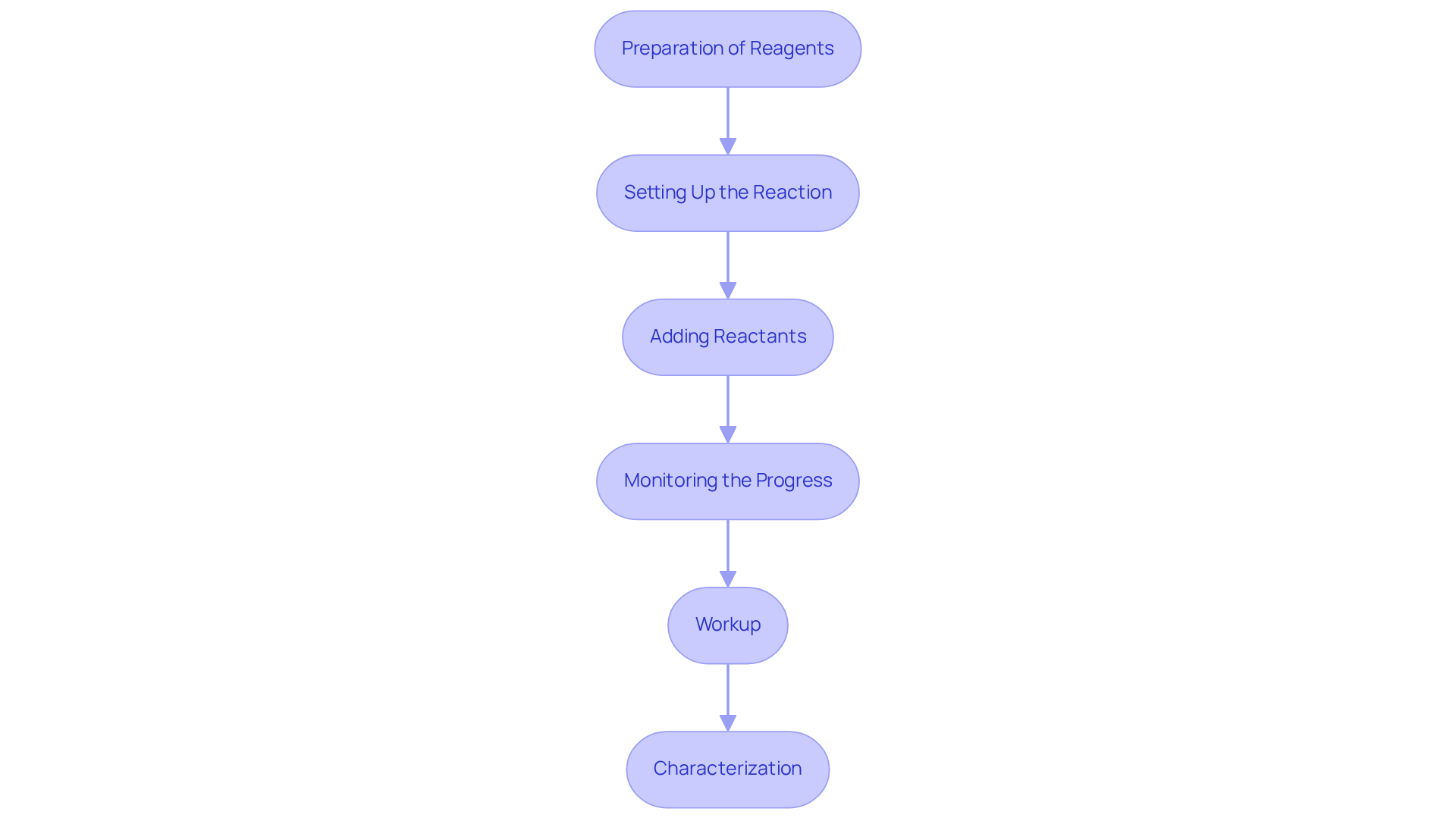
Implement Copper(I) Iodide in Your Synthesis Projects
To effectively incorporate Copper(I) iodide into your synthesis projects, follow these essential steps:
- Preparation of Reagents: Start with high-purity Copper(I) chloride and gather , including aryl halides, alkynes, and solvents such as dimethyl sulfoxide (DMSO) or tetrahydrofuran (THF).
- Setting Up the Reaction: In a fume hood, dissolve the Cu(I) halide in your selected solvent. The concentration typically ranges from 0.1 to 0.5 M, depending on the specific conditions of the process. Notably, the energy transfer efficiency in associated metal-organic frameworks (MOFs) has been shown to exceed 95%, underscoring the potential effectiveness of copper 1 iodide in facilitating reactions.
- Adding Reactants: Gradually introduce your aryl halide and terminal alkyne into the solution. Stir the mixture at room temperature or apply gentle heat as necessary, based on the process conditions.
- Monitoring the Progress: Employ thin-layer chromatography (TLC) to monitor the reaction's advancement, enabling you to determine when completion has been achieved. This step is crucial, as challenges in obtaining reliable instrumentation can significantly affect the accuracy of your monitoring.
- Workup: Upon completion, quench the reaction with water or an appropriate quenching agent. Extract the organic layer and purify the resulting product using column chromatography.
- Characterization: Finally, confirm the structure and purity of your product through characterization techniques such as NMR or mass spectrometry. Experts in the field emphasize that understanding the structure-property relationship is essential for optimizing applications in catalysis and photonics.
By following these comprehensive steps, you can effectively utilize copper 1 iodide in your organic synthesis projects, supported by the innovative products and resources provided by JM Science Inc.
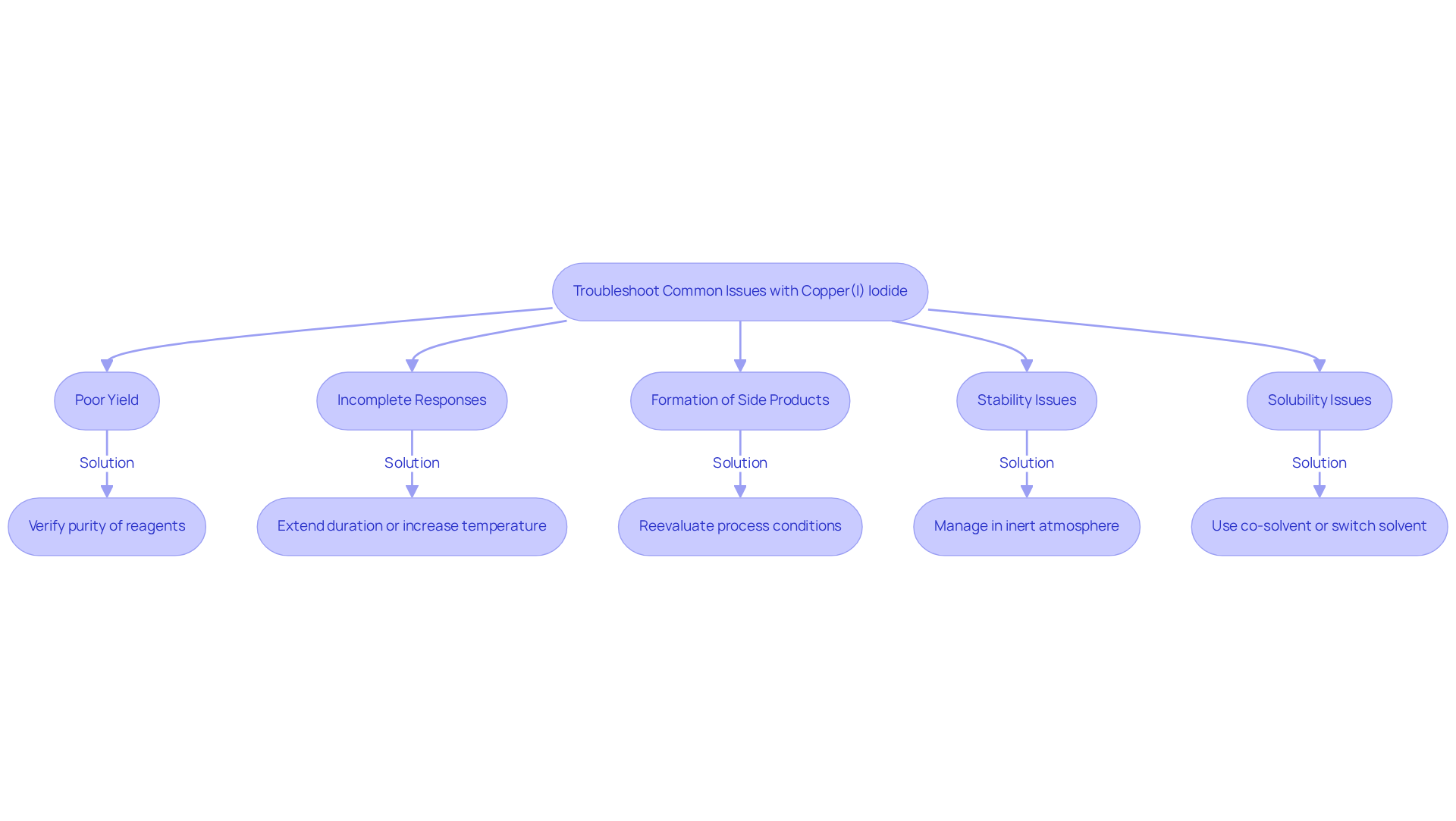
Troubleshoot Common Issues with Copper(I) Iodide
When addressing issues related to Cu(I) halide, several typical problems may arise that can hinder your organic synthesis efforts. Here are some effective troubleshooting tips to consider:
- Poor Yield: If are lower than expected, verify the purity of your copper 1 iodide and other reagents. Impurities can significantly reduce efficiency; studies indicate that only 7 out of 40 processes yielded a pure product when utilizing suboptimal reagents. This underscores the necessity of ensuring high-quality starting materials to enhance yields.
- Incomplete Responses: If the process appears incomplete, consider extending the duration or increasing the temperature. Ensuring thorough mixing of reagents and maintaining an optimal process environment are also vital for achieving desired outcomes.
- Formation of Side Products: Should unexpected side products emerge, reevaluate your process conditions. Adjusting the solvent or altering the concentration of copper 1 iodide can help mitigate side effects. Research has shown that CuI outperformed NaI in challenging processes, achieving nearly complete yields, emphasizing the importance of refining conditions to minimize side products.
- Stability Issues: Cu(I) halide is prone to oxidation to Cu(II) halide when exposed to air. To prevent this, manage it in an inert atmosphere, such as nitrogen or argon, and limit moisture exposure, which can compromise the integrity of your processes.
- Solubility Issues: If Cu(I) halide demonstrates limited solubility in your chosen solvent, consider employing a co-solvent or switching to a more suitable solvent that enhances its solubility. This adjustment can significantly boost reaction efficiency and yield.
By proactively addressing these common challenges, you can improve your success rate with copper 1 iodide in organic synthesis, ultimately leading to more reliable and efficient outcomes.
Conclusion
Mastering the use of copper(I) iodide in organic synthesis unlocks a realm of innovative chemical transformations. This versatile compound serves not only as an effective catalyst in pivotal reactions such as the Sonogashira coupling but also showcases remarkable efficiency in the formation of various organic compounds. A comprehensive understanding of the properties, applications, and methodologies associated with copper(I) iodide is essential for chemists aiming to enhance their synthetic strategies.
The critical properties of copper(I) iodide, including its low solubility, thermal stability, and function as a reducing agent, are detailed herein. These characteristics are crucial for its application in organic synthesis, particularly in cross-coupling processes that facilitate the formation of carbon-carbon bonds. Moreover, practical guidance on preparation, implementation, and troubleshooting common issues underscores the necessity of meticulous handling and optimal conditions to achieve desired outcomes.
In light of these insights, it is evident that leveraging copper(I) iodide can significantly propel organic synthesis endeavors. Chemists are urged to adopt these best practices and refine their techniques to fully harness the potential of this compound. As research continues to advance, embracing such innovative approaches will not only enhance productivity but also contribute to the development of new materials and pharmaceuticals, solidifying copper(I) iodide's role as a cornerstone in modern organic chemistry.




