Overview
This article delves into the preparation, application, and troubleshooting of copper (I) iodide, underscoring its critical role as a semiconductor with diverse applications in laboratory environments. It outlines comprehensive methods for synthesizing CuI, highlights its vital functions in organic synthesis and photocatalysis, and addresses prevalent challenges such as oxidation and low yield. The discussion emphasizes the necessity of proper handling and optimal conditions to preserve its efficacy in experimental settings, reinforcing the significance of meticulous practices in scientific research.
Introduction
Copper (I) iodide stands as a pivotal semiconductor within the domain of materials science, possessing distinctive properties that render it essential for a myriad of electronic applications. This guide explores the preparation, application, and troubleshooting of this multifaceted compound, underscoring its importance in areas such as organic synthesis and photocatalysis.
Nevertheless, despite its remarkable potential, challenges such as oxidation and solubility issues may impede its effectiveness. How can researchers effectively navigate these obstacles to fully leverage the capabilities of copper (I) iodide in their experimental endeavors?
Understand the Properties of Copper (I) Iodide
Copper (I) iodide, also known as Cu(I) halide (CuI), is a significant semiconductor, characterized by its band gap energy of approximately 2.73 eV at a doping concentration of 3 at%. This positions copper (I) iodide as a valuable substance in numerous electronic applications. Typically, this compound manifests as a white or yellowish solid and demonstrates low solubility in water, while being soluble in organic solvents such as acetone and ethanol. As noted by chemists, "The compound (I) Iodine shows considerable solubility in organic solvents, which is essential for its use in various chemical processes." Stability is a critical factor; copper (I) iodide can oxidize to Copper (II) Iodide (CuI2) when exposed to moisture or air, potentially impacting its performance in experimental settings. This oxidation can lead to changes in its electronic characteristics, which is crucial for researchers to consider when employing CuI in sensitive applications.
Recent studies have underscored of copper (I) iodide thin films, which include a transmittance exceeding 97.5% in the visible spectrum and a resistivity that peaks at 1.03 x 10 Ω cm at 3 at% iodine doping. It is important to highlight that this resistivity is the highest observed and varies with different doping levels, influencing the performance of devices utilizing CuI. These attributes render copper (I) iodide a compelling option for photodetectors and solar cells, where its high photocurrent and responsivity have been demonstrated in devices such as the copper (I) iodide/Si junction for UV detection.
The unique properties of copper (I) iodide, including its average work function of approximately 5.6 eV, further enhance its utility in electronic applications. Understanding these properties is essential for effectively handling copper (I) iodide and predicting its behavior in various chemical reactions, particularly in the context of semiconductor technology.
Prepare Copper (I) Iodide for Experiments
To prepare Copper (I) Iodide (CuI), follow these detailed steps:
- Gather Materials: Collect copper(I) chloride (CuCl), potassium iodide (KI), distilled water, and a beaker.
- Dissolve Reactants: In a beaker, dissolve 1 mole of CuCl in 100 mL of distilled water. In a separate container, dissolve 1 mole of KI in 100 mL of distilled water.
- Mix Solutions: Gradually add the KI solution to the CuCl solution while stirring continuously. This will lead to the instant creation of a white precipitate of copper (I) iodide.
- Isolate Copper (I) Iodide: Allow the precipitate to settle, then decant the supernatant liquid. Wash the precipitate with distilled water to eliminate any unreacted materials.
- Dry the Product: Transfer the copper (I) iodide to a drying oven set at 60°C for 2 hours to remove residual moisture. It is crucial to maintain low humidity conditions during this process to prevent oxidation. Keep in a desiccator to prevent additional oxidation.
Recent advancements in the synthesis of copper (I) iodide have shown that the average particle size of copper (I) iodide nanoparticles typically varies from 8 to 12 nm, enhancing their application in various fields such as photovoltaics and biosensing. Yield percentages for this synthesis typically reach around 27.6% purity, demonstrating the effectiveness of this method. Best practices for isolating and drying copper (I) iodide emphasize the importance of maintaining low humidity conditions to prevent oxidation, which can compromise the integrity of the copper (I) iodide product. Laboratory managers recommend thorough washing of the precipitate to ensure high purity levels, as typical student results for copper and iodine are within about 1% and 3% of theoretical values, respectively. As noted by a laboratory manager, 'Thorough washing is essential to achieve the desired purity in the synthesis of copper (I) iodide.' By following these guidelines, researchers can attain consistent and repeatable outcomes in their experiments involving Cu(I) halide.
Apply Copper (I) Iodide in Laboratory Experiments
(CuI) is a compound of considerable versatility, playing a pivotal role in various laboratory experiments. Its applications are extensive and impactful, particularly in the following areas:
- Synthesis of Organometallic Compounds: CuI is an essential catalyst in the synthesis of organometallic compounds, especially in coupling reactions involving aryl halides. Remarkably, this method has achieved product yields of up to 96% in S-Arylation reactions under mild conditions, underscoring the effectiveness of copper (I) iodide in facilitating these reactions, particularly within the frameworks of Negishi and Ullmann Couplings.
- Photocatalysis: Utilizing its semiconductor properties, copper (I) iodide is employed in photocatalytic reactions, particularly in the degradation of organic pollutants when subjected to light. Research indicates that copper (I) iodide can significantly enhance the degradation efficiency of various organic compounds, emphasizing its vital role in sustainable chemistry and environmental remediation.
- Preparation of Nanomaterials: Copper (I) iodide serves as a precursor for the synthesis of copper-based nanomaterials, which are increasingly sought after in electronics and photonics. The ability to control the size and shape of these nanomaterials enhances their applicability across numerous fields, thereby establishing copper (I) iodide as a crucial component in nanotechnology.
- Organic Synthesis: In the realm of organic synthesis, copper (I) iodide is integral to forming carbon-carbon (C-C) bonds, which is a fundamental step in pharmaceutical development. The significance of copper (I) iodide is exemplified by its involvement in reactions such as the Sonogashira Coupling, which generates valuable intermediates for drug discovery. Moreover, copper (I) iodide's capacity to facilitate these reactions under mild conditions further accentuates its utility in pharmaceutical contexts.
These applications underscore the essential role of copper (I) iodide in advancing laboratory research and highlight its potential to drive innovation across various scientific domains. However, it is crucial to acknowledge potential limitations, such as solubility challenges and the necessity for specific reaction conditions, to fully grasp the applicability of copper (I) iodide in laboratory environments.
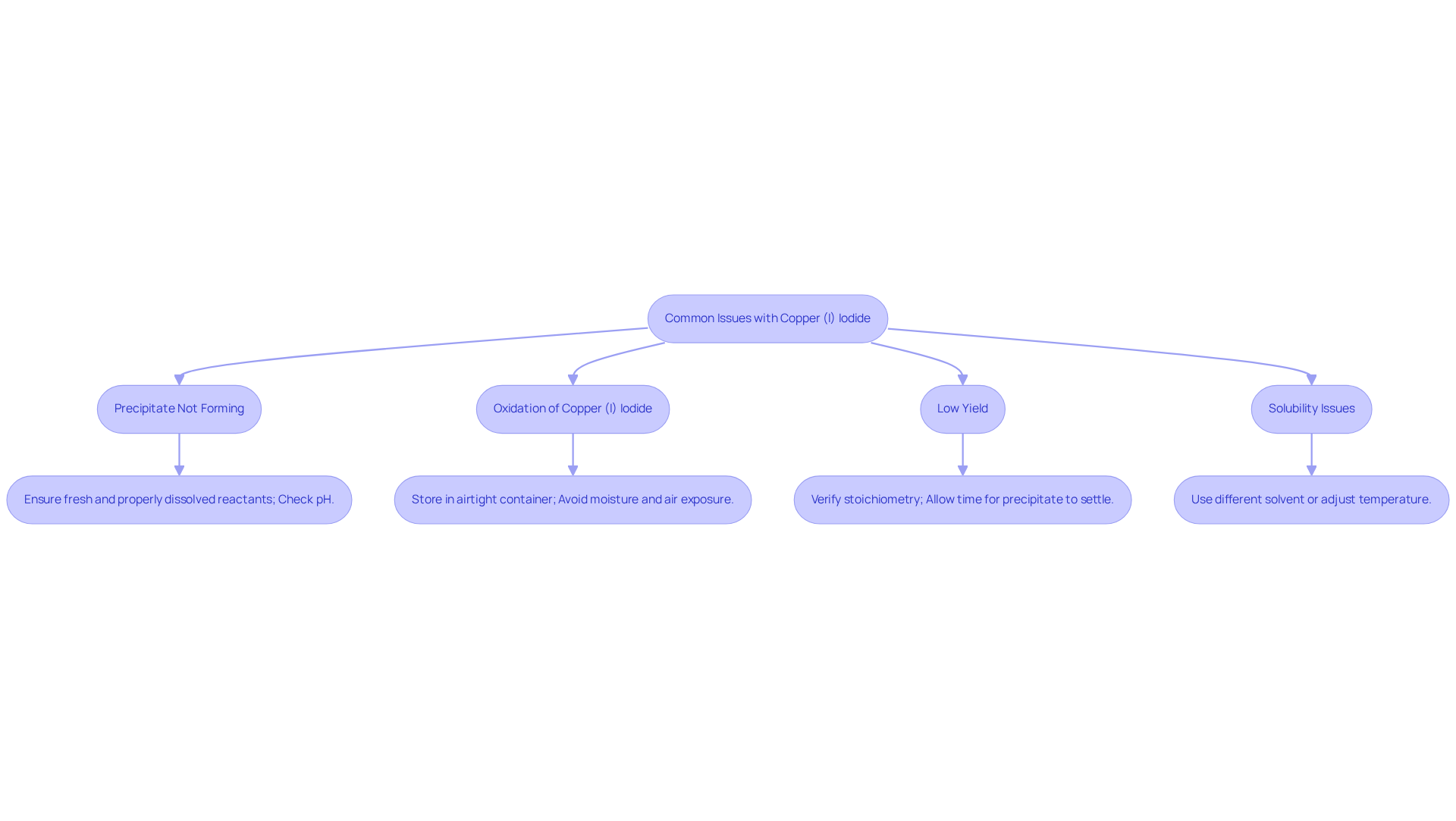
Troubleshoot Common Issues with Copper (I) Iodide
When working with Copper (I) Iodide, several issues may arise that require attention:
- Precipitate Not Forming: If no precipitate forms during the mixing of CuCl and KI, it is essential to ensure that both reactants are fresh and properly dissolved. Additionally, check the pH of the solution, as extreme pH levels can inhibit precipitation.
- Oxidation of Copper (I) Iodide: Copper (I) Iodide is a compound of interest in various chemical applications. The oxidation of Copper (I) Iodide may result in a brown or dark appearance, indicating its conversion to Copper (II) Iodide. To prevent this, store CuI in an airtight container and under inert conditions, avoiding exposure to moisture and air.
- Low Yield: Should the yield of copper (I) iodide (CuI) be lower than expected, verify the stoichiometry of your reactants. Allow sufficient time for the precipitate to settle to ensure a complete reaction.
- Solubility Issues: If copper (I) iodide is not dissolving in your solvent, consider using or adjusting the temperature to enhance solubility.
Addressing these issues effectively will ensure optimal results when working with copper (I) iodide.
Conclusion
Copper (I) iodide (CuI) emerges as a pivotal compound in the realm of chemistry, showcasing extensive utility across various applications due to its unique properties. As a semiconductor, it possesses remarkable optical and electrical characteristics, and its versatility in facilitating chemical reactions positions copper (I) iodide as a significant material in both laboratory settings and industrial applications.
This article delves into the preparation methods, highlighting essential steps to synthesize CuI effectively, while emphasizing the importance of maintaining low humidity conditions to prevent oxidation. Furthermore, it explores the diverse applications of copper (I) iodide, including its critical role in organic synthesis, photocatalysis, and nanomaterial preparation. Troubleshooting common issues, such as precipitation failures and oxidation concerns, provides valuable insights for researchers aiming to optimize their experiments.
Ultimately, the significance of copper (I) iodide cannot be overstated. Its applications drive advancements in scientific research and pave the way for innovative solutions in technology and environmental sustainability. As the understanding of this compound deepens, opportunities for its utilization in various fields will undoubtedly expand, encouraging further exploration and application of copper (I) iodide in modern chemistry.




