Overview
The article highlights the pivotal role and configuration of counter electrodes in laboratory experiments, particularly within electrochemical cells. Counter electrodes are indispensable for completing electrical circuits, thereby ensuring precise measurements. Selecting suitable materials and configurations is crucial; this choice significantly enhances both experimental reliability and performance. Understanding these elements is essential for researchers aiming to optimize their experimental outcomes, ultimately leading to more accurate and reproducible results.
Introduction
In the intricate realm of electrochemical experiments, counter electrodes emerge as pivotal components, steadfastly ensuring the stability and accuracy of measurements that propel scientific discovery. These auxiliary electrodes are indispensable in completing electrical circuits, facilitating the seamless flow of current essential for electrochemical reactions.
As technological advancements push the frontiers of research, the selection and design of counter electrodes have evolved, highlighting not only their functionality but also sustainability and material integrity. From platinum and gold to innovative carbon-based and conductive polymer options, the diverse landscape of counter electrodes is meticulously tailored to meet specific experimental needs.
This article explores the critical role of counter electrodes, examining their types, applications, and best practices for setup, ultimately underscoring their significance in advancing pharmaceutical research and electrochemical studies.
Define Counter Electrodes and Their Functionality
Counter electrodes, which are also referred to as auxiliary terminals, are pivotal in chemical cells, particularly within three-terminal configurations. Their primary function is to complete the electrical circuit, facilitating current flow between the active component and the external circuit. This current flow is vital for maintaining the stability of the chemical reactions occurring at the operating terminal.
To ensure accurate measurements, supporting components must be constructed from materials that can endure the chemical environment without engaging in undesirable side reactions. This property is crucial to avoid interference with the measurements. Commonly used materials include platinum, gold, and various carbon-based compounds, all selected for their inertness and exceptional conductivity.
The significance of counter components extends beyond mere functionality; they are fundamental to the reliability of chemical experiments. Recent advancements in sensor design emphasize sustainability and the use of inert materials to enhance measurement accuracy. As highlighted in the case study "Challenges in Electrochemical Measurements," optimizing configuration setups can mitigate interference from reaction products, thus improving the reliability of electrochemical assessments. Furthermore, a researcher asserts that 'sustainability in design is not merely a trend; it’s essential for responsible scientific progress.'
While this discussion centers on three-electrode setups, it is also crucial to recognize the importance of two-electrode setups, especially when the counter electrode potential is anticipated to remain stable throughout experiments. As the field evolves, the integration of opposing terminals with other analytical techniques continues to enhance performance, underscoring their importance in modern laboratory settings. For pharmaceutical lab managers, the reliability and precision of counter devices directly impact research and development processes, making their careful selection and configuration imperative.
Explore Types of Counter Electrodes and Their Applications
Counter terminals are pivotal in chemical experiments, with various types tailored for specific applications. Below are the primary categories:
- Platinum Conductors: Known for their outstanding conductivity and inertness, platinum conductors are the preferred choice for a broad spectrum of electrochemical applications. However, their suitability in two-compartment cells hinges on specific conditions, such as maintaining a controlled environment and ensuring proper electrolyte composition.
- Gold Contacts: These contacts excel in delicate measurements due to their stability and ability to form self-assembled monolayers. This characteristic is particularly beneficial in biosensing applications, where precision is critical.
- Carbon-Based Conductors: Comprising glassy carbon and carbon nanotubes, these materials are prized for their high surface area and efficacy in facilitating electron transfer. Their increasing use in energy storage devices underscores their versatility in contemporary applications.
- Conductive Polymers: Employed in specific applications like organic solar cells, conductive polymers leverage their unique properties to enhance performance, showcasing the variety of alternative materials available.
Recent technological advancements have highlighted substitutes for conventional components. For instance, a quantitative framework has been established to identify suitable adsorption energy ranges for active surfaces, confirming that alternatives such as CoS and MoC can effectively replace platinum in certain chemical processes. Furthermore, studies suggest that α-Fe may serve as a promising catalyst in dye-sensitized solar cells (DSCs), potentially decreasing dependence on costly platinum. Notably, research indicates that platinum substances undergo (electro)dissolution during repeated potential cycling, with investigations documenting up to 5000 transients, raising concerns regarding their long-term stability, which is influenced by the choice of counter electrode based on the specific requirements of the experiment, including the type of electrolyte and the intended reaction kinetics.
Understanding these factors is essential for optimizing performance and advancing research in pharmaceutical applications. As Gregory Jerkiewicz emphasized, progress in alternative material types is vital for enhancing the efficiency and effectiveness of electrochemical processes across various sectors, including pharmaceuticals. This underscores the importance of selecting the appropriate alternative to meet the specific needs of pharmaceutical research.
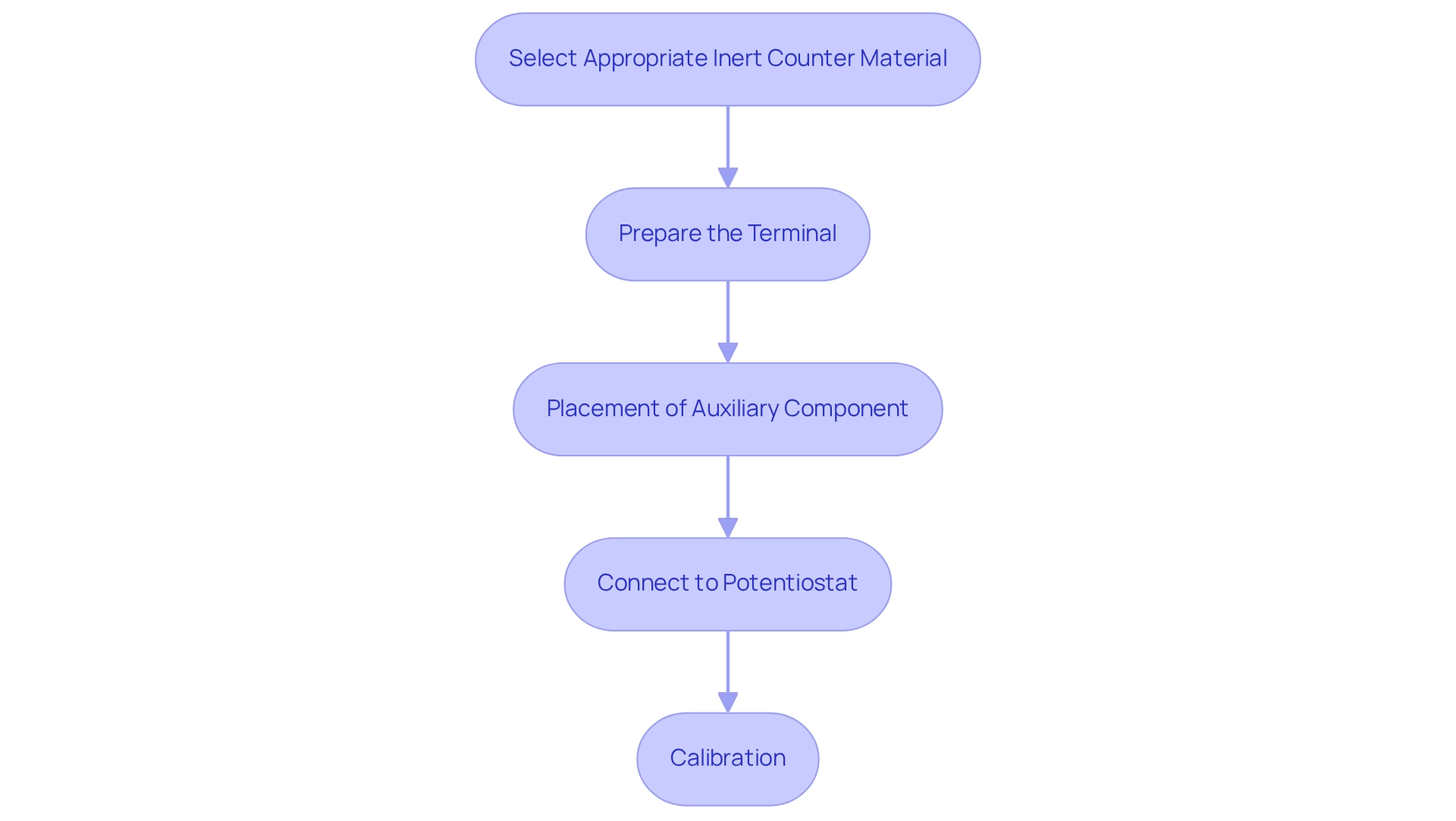
Set Up Counter Electrodes for Optimal Performance
To achieve optimal performance with counter electrodes, it is crucial to adhere to best practices that ensure reliability and efficiency in electrochemical experiments, starting with the selection of an appropriate inert counter material that is compatible with your electrolyte solution. This selection is vital for ensuring stability and reliability. For instance, the nickel–titanium alloy component prepared by Pang has demonstrated higher charge transfer and electrocatalytic activity, making it a strong candidate for various applications.
Prepare the Terminal: Thoroughly clean the terminal surface to eliminate contaminants that could compromise performance. Utilizing distilled water and a soft cloth will ensure effective cleaning, setting the stage for accurate measurements.
Placement: Arrange the auxiliary component at an optimal distance from the working terminal to reduce resistance and promote efficient current flow. It is essential to consider the geometry of the electrochemical cell during this process. Customizing complementary terminals, including the counter electrode, for specific conditions in anode and cathode LSV tests is critical for obtaining optimal outcomes.
Connect to the Potentiostat: Ensure the reference terminal is properly connected to the potentiostat, following the manufacturer’s wiring guidelines to maintain system integrity.
Calibration: Prior to experimentation, calibrate the setup using known standards to ensure measurement accuracy. This may involve performing a cyclic voltammetry test to verify the response of the secondary conductor.
Real-world applications illustrate that accurate positioning and substance selection significantly influence electrochemical performance. For example, advancements in opposing material types for dye-sensitized solar cells (DSSC) underscore the importance of composite materials that enhance efficiency and stability. Research indicates that customized supplementary components can improve photovoltaic performance, with a documented photoelectric energy conversion efficiency of 6.40% under specific pulse parameters. This finding highlights a promising path for future investigations. By adhering to these procedures, laboratories can optimize their auxiliary component arrangements, resulting in more dependable and effective electrochemical experiments.
Conduct Experiments Using Counter Electrodes: A Step-by-Step Guide
To effectively conduct experiments utilizing counter electrodes, follow this comprehensive step-by-step guide:
- Prepare the Electrochemical Cell: Assemble a three-electrode system, ensuring the working component, reference component, and auxiliary element are securely positioned within the electrolyte solution. This setup is crucial for accurate measurements and reliable data, as the complex physical phenomena guiding cell performance can significantly influence outcomes.
- Set up the potentiostat by connecting the electrodes, making sure the counter electrode is linked to the correct terminal. Proper connections are essential for the potentiostat to function effectively and provide accurate readings.
- Select Experimental Parameters: Choose parameters tailored to your specific chemical reaction, including scan rate, potential range, and duration. These settings significantly influence the outcome and quality of your data. For instance, studies have shown that adjusting the desorption rate of HCOO* species on surfaces can significantly impact reaction kinetics, underscoring the need for precise control in experimental setups.
- Run the Experiment: Initiate the potentiostat and monitor data collection closely. Observe the current response and potential changes throughout the experiment, as these metrics are vital for understanding the chemical processes at play.
- Examine the Data: After completing the experiment, analyze the gathered data to interpret the observed chemical behavior. Focus on key indicators such as peak currents and potential shifts, which can reveal insights into reaction kinetics and efficiency. As Dr. Weiran Zheng noted, after collecting the spectra, researchers are required to process and present the data to support their claims.
- Troubleshoot as Necessary: If unexpected results arise, review your setup and data for potential issues, such as contamination of the sensors or improper connections. Addressing these factors is critical for ensuring the reliability of your experimental outcomes.
Recent trends in chemical experimentation emphasize the importance of optimizing potentiostat settings to enhance data quality. Furthermore, there is a growing need for further studies on the overall efficiency of hydrogen production systems, considering balance-of-plant implications. By adhering to this guide, researchers can effectively utilize counter electrodes to advance their electrochemical studies, as demonstrated in the case study on 'Water in Electrocatalysis,' which emphasizes how water plays a role in enhancing reaction kinetics.
Conclusion
Counter electrodes are vital components in electrochemical experiments, serving to complete electrical circuits and ensure the accuracy and reliability of measurements. The exploration of various types of counter electrodes—from platinum and gold to innovative carbon-based and conductive polymer materials—reveals a rich landscape of options tailored for specific applications. Each material presents unique benefits and challenges, underscoring the importance of careful selection based on experimental needs.
The setup and execution of experiments utilizing counter electrodes necessitate adherence to best practices to achieve optimal performance. Factors such as material selection, electrode positioning, and calibration play crucial roles in minimizing interference and enhancing measurement accuracy. By following a structured approach to experimental design, researchers can significantly improve the reliability and efficacy of their electrochemical studies.
Ultimately, advancements in counter electrode technologies and materials highlight their indispensable role in pharmaceutical research and electrochemical applications. As the field evolves, the emphasis on sustainability and innovation in electrode design will be paramount. By prioritizing the selection and configuration of counter electrodes, researchers can drive scientific discovery and contribute to the advancement of electrochemical processes, paving the way for future breakthroughs across various scientific disciplines.




