Overview
This article serves as an authoritative step-by-step guide for lab managers aiming to master cross titration. It underscores the critical need to grasp fundamental principles, assemble the requisite equipment, execute the procedure with precision, and troubleshoot prevalent issues. Detailed explanations of concepts such as equivalence points, indicator selection, and the importance of accurate measurements are provided. This comprehensive guidance empowers lab managers to significantly enhance the reliability and reproducibility of their titration assays.
Introduction
Mastering the art of cross titration is essential for lab managers who seek to ensure accurate and reliable analytical results. This intricate technique not only aids in determining unknown concentrations but also enhances compliance with international standards. However, the journey toward precision is fraught with challenges, including the selection of appropriate indicators and the avoidance of common measurement pitfalls.
How can lab managers effectively navigate these complexities to achieve consistent and trustworthy outcomes in their experiments?
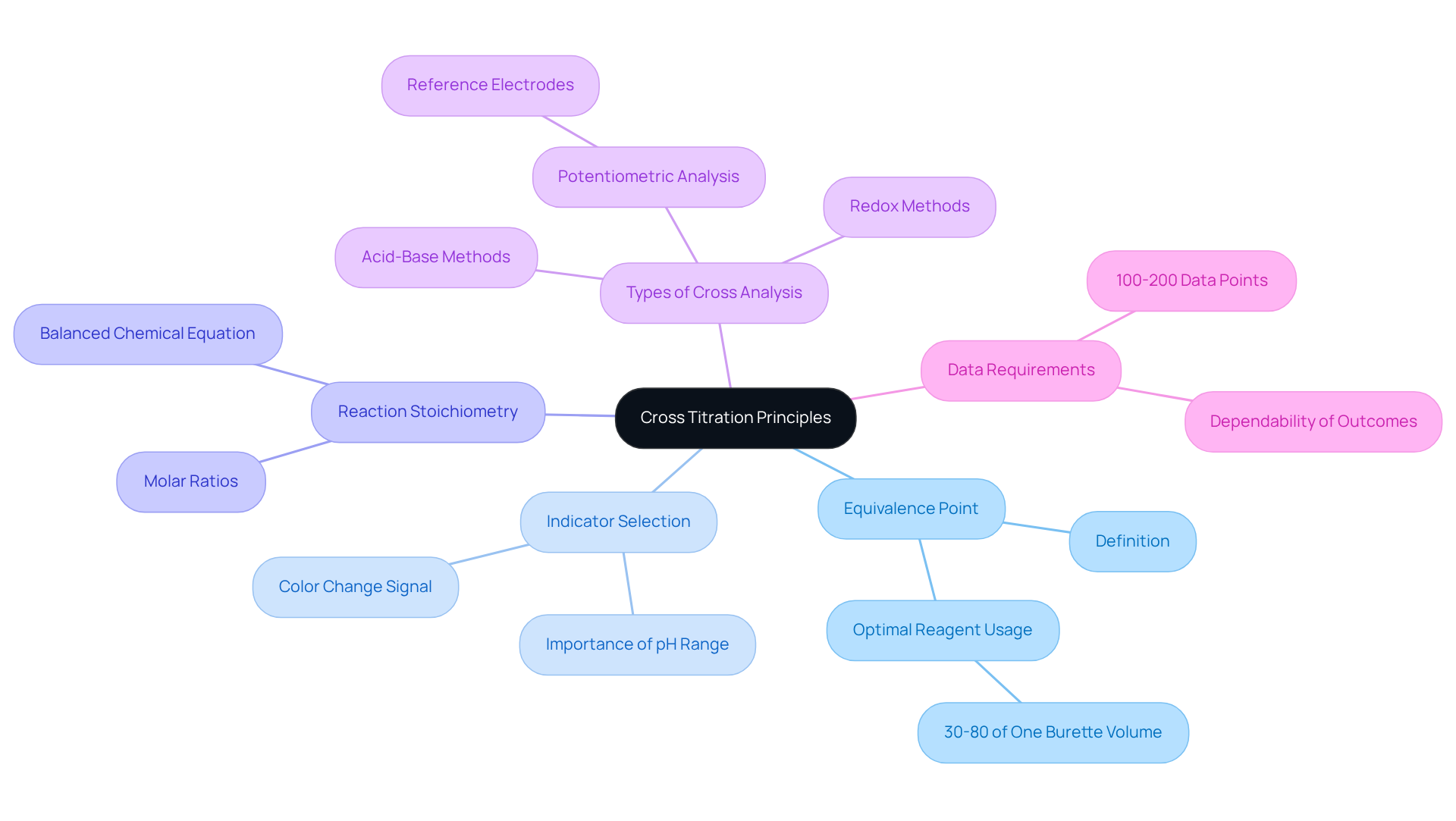
Understand Cross Titration Principles
Cross analysis serves as a vital technique for determining the concentration of an unknown solution using a second solution of known concentration, especially when direct measurement of the analyte proves challenging. Understanding key principles is essential:
-
Equivalence Point: This pivotal moment occurs when the quantity of reagent added is stoichiometrically equivalent to the substance amount in the sample. Mastery of this concept is crucial for accurately determining the endpoint of the process. Research indicates that an optimal procedure should achieve a reagent usage of 30 to 80% of one burette volume, ensuring accuracy in outcomes. Various studies support this statistic, highlighting the importance of optimal titrant consumption for reliable results.
-
Indicator Selection: The choice of indicator is critical, as it signals the endpoint of the process through a distinct color change. The selection process must consider the reaction's pH range to guarantee optimal performance.
-
Reaction Stoichiometry: A comprehensive understanding of the balanced chemical equation governing the reaction is necessary for accurate concentration calculations. This includes knowledge of the molar ratios between the involved reactants, which is vital for achieving reliable results.
-
Types of Cross Analysis: Familiarity with various types of cross analysis, such as acid-base and redox methods, enhances experimental planning and execution. For instance, in potentiometric analysis, reference electrodes are employed to ensure precise measurements, underscoring the significance of the equivalence point.
-
Data Requirements: It is essential to note that cross-titration experiments generally require 100-200 data points to ensure dependable outcomes. This data requirement is crucial for lab managers to consider when designing their experiments.
By mastering these principles, lab managers can significantly enhance the reliability and reproducibility of their cross titration assays, ultimately contributing to improved performance and compliance with international standards. Moreover, recognizing the importance of repeatability in clinical studies will further bolster the integrity of their results.
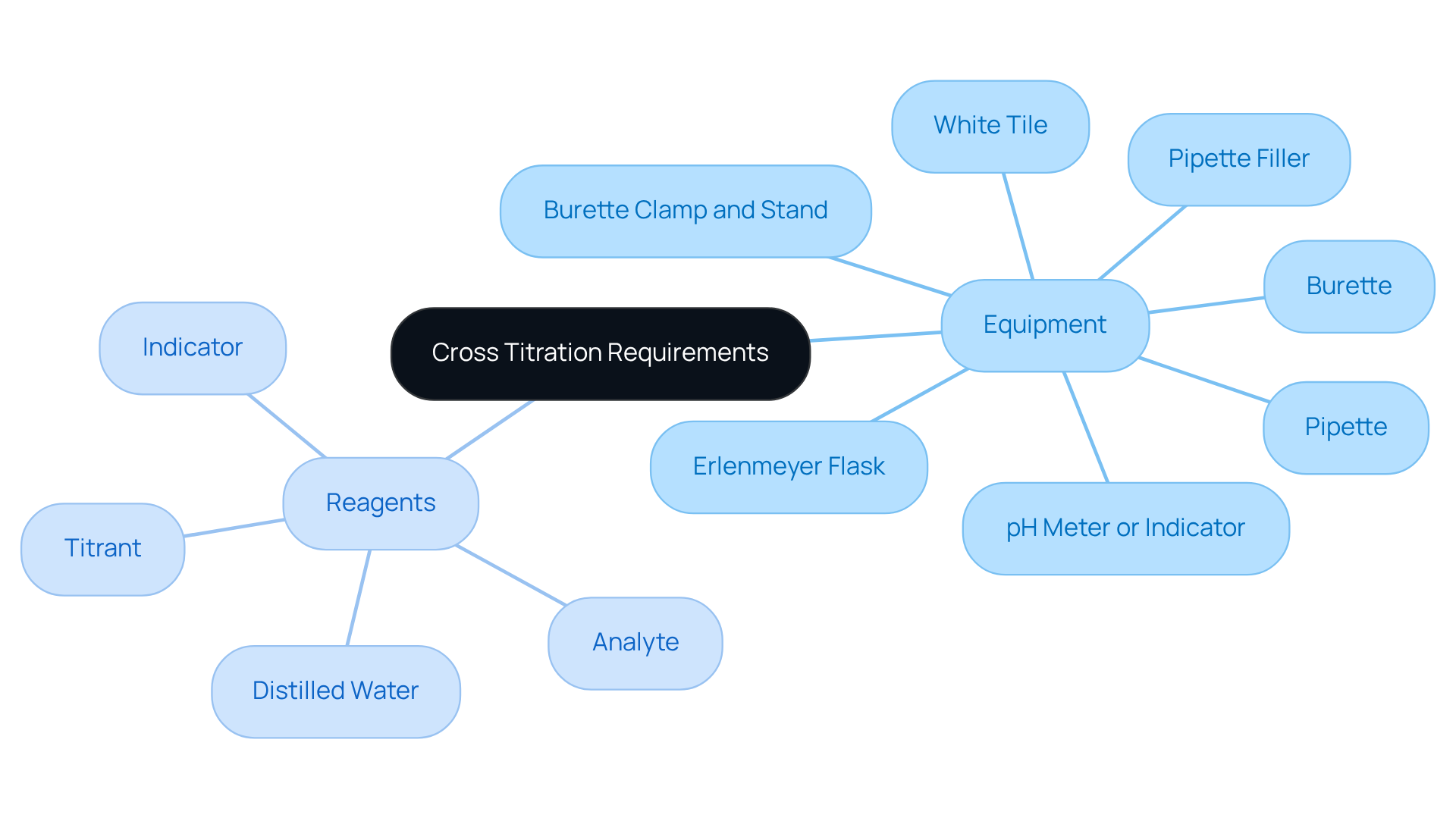
Gather Required Equipment and Reagents
To effectively conduct cross titration, assembling the following equipment and reagents is essential:
Equipment:
- Burette: Essential for accurately dispensing the titrant.
- Pipette: Used for precise measurement of the analyte solution.
- Erlenmeyer Flask: Functions as the vessel for the analyte during the analysis.
- Pipette Filler: Ensures safe and accurate filling of the pipette.
- Burette Clamp and Stand: Provides secure support for the burette.
- White Tile: Helps in noticing color changes during the measurement process.
- pH Meter or Indicator: Essential for identifying the endpoint of the process.
Reagents:
- Titrant: A solution with a known concentration, such as NaOH for acid-base titrations.
- Analyte: The solution whose concentration needs to be determined.
- Indicator: A substance that alters hue at the endpoint, such as phenolphthalein for acid-base analyses.
- Distilled Water: Necessary for dilutions and rinsing equipment.
Before commencing the experiment, ensure that all equipment is thoroughly cleaned and calibrated. This practice is essential to avoid contamination and guarantee precise outcomes. Furthermore, the significance of utilizing calibrated instruments greatly decreases the likelihood of mistakes in measurement outcomes.
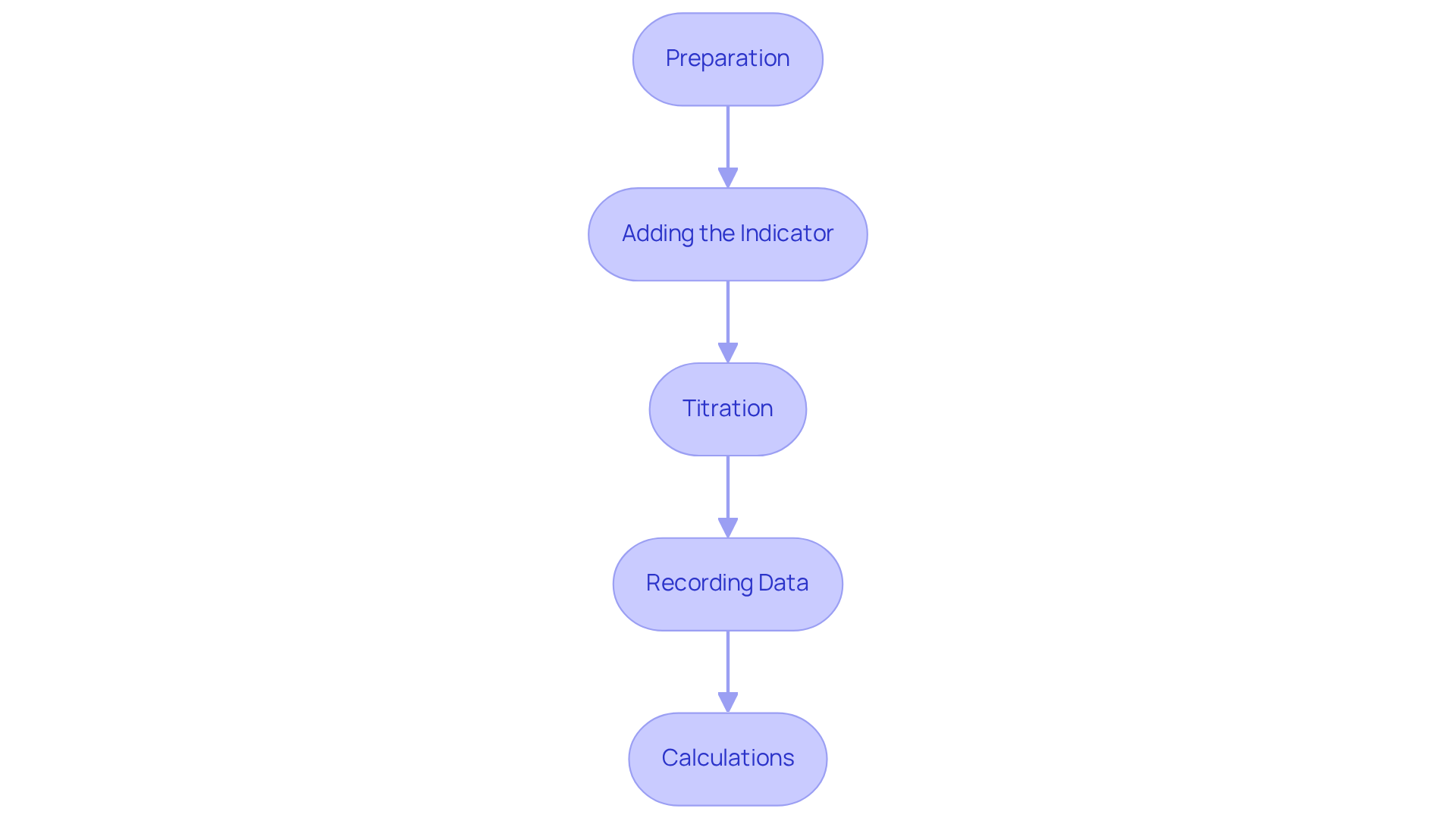
Execute the Cross Titration Procedure
To execute the cross titration procedure effectively, it is essential to follow these steps:
-
Preparation: Begin by thoroughly cleaning all glassware to prevent contamination; even minor residues can skew results. Regular calibration of equipment is crucial for ensuring accuracy and consistency in titration results. Rinse the burette with the titrant liquid and fill it, ensuring no air bubbles are present, as they can lead to inaccuracies in volume measurement. Utilize a pipette to measure a specific volume of the analyte liquid and transfer it to the Erlenmeyer flask.
-
Adding the Indicator: Introduce a few drops of the chosen indicator to the analyte solution in the flask. Selecting an appropriate indicator is crucial; it should provide a sharp color change at the expected endpoint. As Robert Bunsen stated, "The selection of the indicator is as significant as the selection of the reagent."
-
Titration: Position the Erlenmeyer flask on a white tile beneath the burette for better visibility of color changes. Gradually add the reagent from the burette to the analyte while continuously swirling the flask to ensure thorough mixing. Monitor the solution for a color change, indicating that you are nearing the endpoint. As you approach this point, add the reagent dropwise until the color change remains stable.
-
Recording Data: Carefully note the final volume of titrant used from the burette, as this measurement is essential for calculating the concentration of the analyte. Conducting multiple titrations—at least three—is recommended to enhance the accuracy and reliability of results. For instance, a quality control laboratory discovered that quick addition of reagent led to a 10% overestimation of acetic acid concentration, which significantly affected product formulation.
-
Calculations: Utilize the recorded data to calculate the concentration of the analyte using the formula:
C1V1 = C2V2
where C1 and V1 represent the concentration and volume of the titrant, while C2 and V2 denote the concentration and volume of the analyte. This calculation is essential for establishing the precision of your analysis outcomes.
Frequent errors in cross titration include the quick addition of titrant, which can overshoot the endpoint, and a poor choice of indicators, resulting in misinterpretation of results. By adhering to these steps and being mindful of potential mistakes, you can achieve high precision in your experiments.
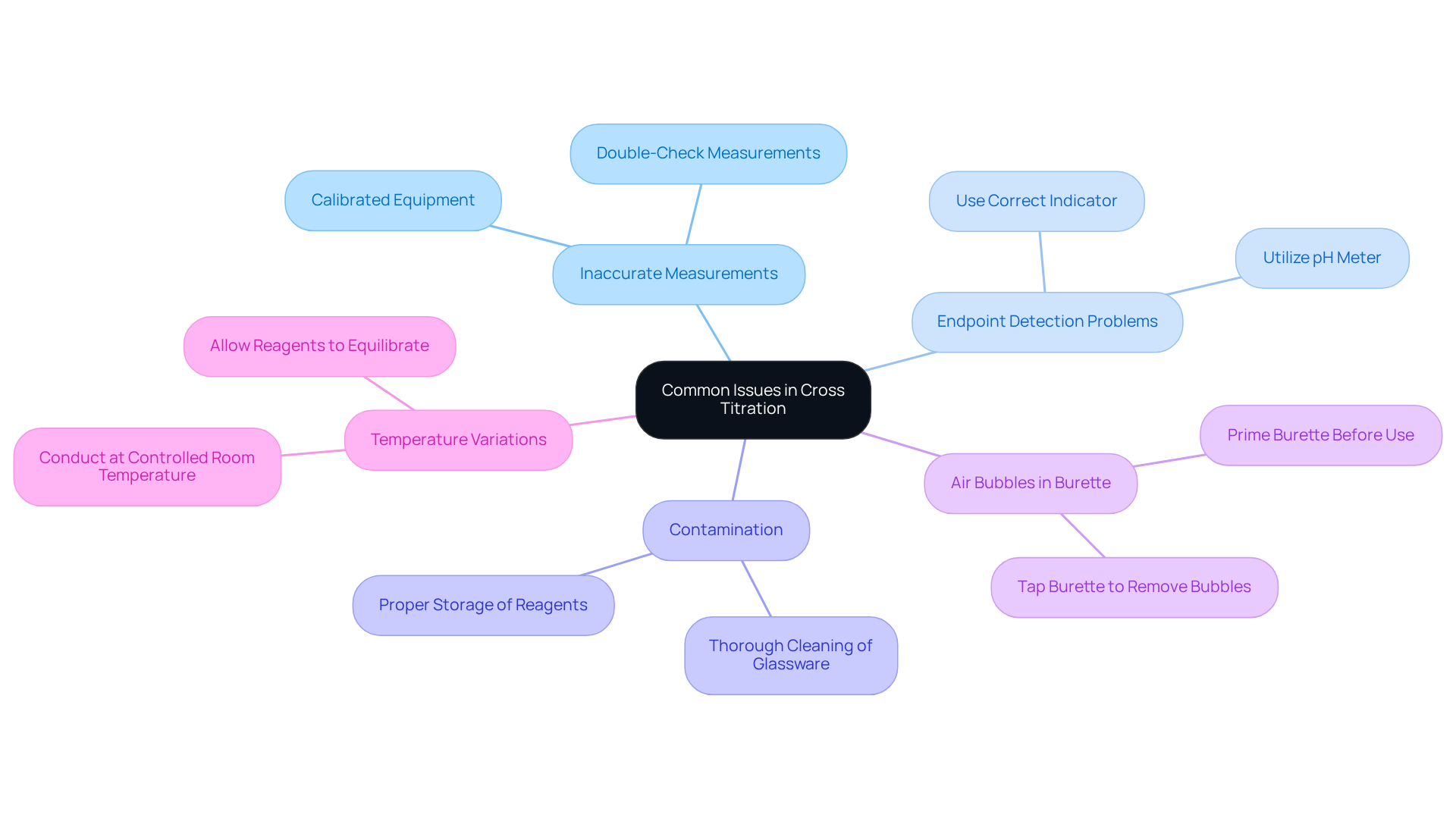
Troubleshoot Common Issues in Cross Titration
Common issues that may arise during cross titration and their solutions are critical for ensuring reliable results in laboratory settings.
Inaccurate Measurements present a significant challenge. When the titrant or analyte volumes are not measured accurately, the results can be skewed, leading to substantial errors. To mitigate this, it is essential to utilize calibrated equipment and double-check measurements prior to proceeding. Regular calibration is crucial, as poorly maintained equipment can result in volume discrepancies of up to ±0.2 mL. For instance, employing a 50 mL burette typically comes with a tolerance of ±0.05 mL, emphasizing the necessity for precision in measurement.
Another prevalent issue is Endpoint Detection Problems. Difficulty in determining the endpoint can lead to over-titration or under-titration, ultimately compromising the accuracy of the results. To address this, ensure the correct indicator is utilized and practice observing the color change. For example, the appropriate indicator for TRIS with HCl should change color at approximately pH 5. Additionally, using a pH meter can enhance endpoint detection accuracy, significantly reducing the likelihood of errors, which are often overlooked in the process.
Contamination poses yet another risk. Contaminated glassware can introduce errors in titration results, leading to unreliable data. To prevent this, it is vital to clean all glassware thoroughly before use and avoid touching the insides of flasks and pipettes. Proper storage of reagents and regularly checking expiry dates are also essential measures to avert contamination.
Air Bubbles in Burette can disrupt accurate titrant volume readings, adversely affecting overall results. To counter this, it is advisable to prime the burette before use to eliminate any air bubbles and ensure a smooth flow of titrant. This practice is critical, as air bubbles can significantly alter the volume dispensed.
Lastly, Temperature Variations can influence reaction rates and the solubility of reagents, leading to inconsistent results. Conducting volumetric analyses at a controlled room temperature and allowing reagents to equilibrate before use is fundamental. For instance, at 20 °C, 1.000 L of n-hexane expands to 1.007 L at 25 °C, resulting in an error of 0.7%. Maintaining a stable temperature is crucial, as even minor shifts can impact the precision of measurements by altering the solubility of the substances involved.
By recognizing these common issues and implementing their respective solutions, lab managers can significantly enhance the reliability of results obtained through cross titration, ensuring more accurate and reproducible outcomes.
Conclusion
Mastering cross titration is essential for lab managers who aim to enhance the accuracy and reliability of their analytical results. By understanding foundational principles, gathering necessary equipment and reagents, executing procedures meticulously, and troubleshooting common issues, one can significantly improve the quality of titration outcomes. This comprehensive approach not only ensures compliance with international standards but also bolsters the integrity of experimental data.
Key insights discussed include:
- The importance of the equivalence point
- The careful selection of indicators
- The necessity for precise measurements
Achieving optimal titrant consumption and understanding reaction stoichiometry are critical for reliable concentration calculations. Furthermore, addressing common pitfalls such as endpoint detection problems and contamination can lead to more reproducible and accurate results in cross titration experiments.
In summary, the effective execution of cross titration requires a combination of theoretical knowledge and practical skills. Lab managers are encouraged to implement best practices and remain vigilant about potential errors, ensuring that their results stand up to scrutiny. By fostering a culture of precision and thoroughness in laboratory settings, they can not only enhance their own operations but also contribute to the broader field of analytical chemistry.




