Overview
This article presents a comprehensive step-by-step guide to mastering EDTA titration. It emphasizes the critical importance of precise pH control, accurate measurement techniques, and the selection of appropriate indicators for achieving reliable results.
- Detailed procedural instructions support this guidance, along with troubleshooting tips for common issues encountered during the process.
- Furthermore, the significance of systematic practice in analytical chemistry is highlighted, ensuring that users can effectively determine metal ion concentrations.
Introduction
In the realm of analytical chemistry, the precision of titration methods can mean the difference between success and failure in experiments. EDTA titration, recognized for its capacity to form stable complexes with metal ions, serves as a cornerstone technique for determining the concentration of these ions in various solutions.
However, mastering this method requires more than just a basic understanding; it necessitates a keen awareness of critical factors such as:
- pH control
- The selection of indicators
- Troubleshooting common pitfalls
From the essential role of maintaining optimal pH levels to the intricacies of choosing the right materials and equipment, this article delves into the fundamental components of EDTA titration, offering a comprehensive guide for both novice and experienced chemists alike.
Understand the Basics of EDTA Titration
Ethylenediaminetetraacetic acid is a vital chelating agent, forming stable complexes with metal ions, which is essential in analytical chemistry. This process utilizes a chelating agent to determine the concentration of metal ions in a solution by reacting with them to form a colored complex. The endpoint is indicated by a distinct color change, signifying that all metal ions have reacted with the EDTA.
The pH at which the analysis is conducted plays a crucial role in the stability of the metal-EDTA complex. Maintaining a pH around 10 is optimal, as it enhances the formation of stable complexes, ensuring accurate results. Recent studies emphasize that the stability of these complexes can significantly fluctuate with pH, underscoring the importance of precise pH control during measurement processes. Expert opinions highlight the critical nature of pH in metal ion complex formation, with chemists asserting that proper pH management is essential for achieving reliable analytical results.
Statistics reveal that 23% of students effectively articulated the reasons for the blue hue observed during the process, linking it to the underlying chemical interactions. This understanding is further reinforced by case studies, such as the investigation into the kinetics of ligand exchange reactions involving lead and copper complexes, which provided insights into the acidity dependence of these reactions and the influence of various ions on reaction rates. As James Doble from the Chemistry and Biochemistry Department at the University of Minnesota Duluth noted, "This activity provides instructors with additional resources to facilitate students’ learning of Le Châtelier’s Principle."
To enhance the procedures, it is advisable to shorten the experiment by decreasing the number of tests for known lead concentrations. Typically, students conduct three trials with a known lead(II) ion concentration before executing three experiments with an unknown lead(II) ion concentration, emphasizing the significance of systematic methods in the process. The use of ethylenediaminetetraacetic acid as a chelating agent in EDTA titration extends beyond mere measurement; it is extensively employed in laboratories for its capacity to bind metal ions, thus improving the precision of various analytical techniques. Mastering the fundamentals of this process is essential for accuracy in analytical chemistry.
Gather Required Materials and Equipment
- EDTA Solution: Employ a standardized solution of ethylenediaminetetraacetic acid, typically at concentrations of 0.01 M or 0.1 M, to ensure accuracy in the measurement process. This precision is critical for reliable outcomes in your analysis.
- Burette: An essential tool for dispensing the solution with precision, the burette allows for controlled addition during the measurement process, enhancing the accuracy of your results.
- Pipette: Utilize a pipette to accurately measure the sample solution, ensuring a consistent volume that is crucial for reliable results. Consistency in measurement is key to achieving valid conclusions.
- Conical Flask: This vessel is necessary for holding the sample during measurement, facilitating easy mixing and observation of color changes. Its design supports effective analysis and observation.
- pH Buffer Solution: It is imperative to maintain the pH around 10 using a suitable buffer solution, as this is critical for the effectiveness of the measurement process. Proper pH levels are essential for accurate readings.
- Indicator: Employ indicators such as Eriochrome Black T or Calmagite, which provide a clear color change at the endpoint, aiding in the accurate determination of the completion of the measurement process. The clarity of these indicators is vital for precise analysis.
- Stirring Rod: A stirring rod is important for thoroughly mixing the solutions, ensuring homogeneity during measurement. This step is crucial for obtaining consistent results.
- White Tile: Place a white tile under the flask to enhance the visibility of the color change, making it easier to identify the endpoint. This simple addition can significantly improve your observation accuracy.
To maintain the integrity of your results, ensure that all glassware is meticulously cleaned and rinsed with the respective solutions prior to use. This practice prevents contamination that could skew your findings and ensures reliable outcomes.
Additionally, for timely procurement of materials, be aware that orders for in-stock items eligible for same-day shipping must be received before 2:00 p.m. local time. Understanding water hardness is essential in treating and managing water, which makes EDTA titration a relevant method for ensuring water quality. While complexometric analysis is the most common method for measuring water hardness, exploring alternative approaches such as ion chromatography and atomic absorption spectroscopy can also be advantageous, depending on specific needs. Efficient ordering processes, such as receiving confirmation emails for quote requests, can further streamline your laboratory operations.
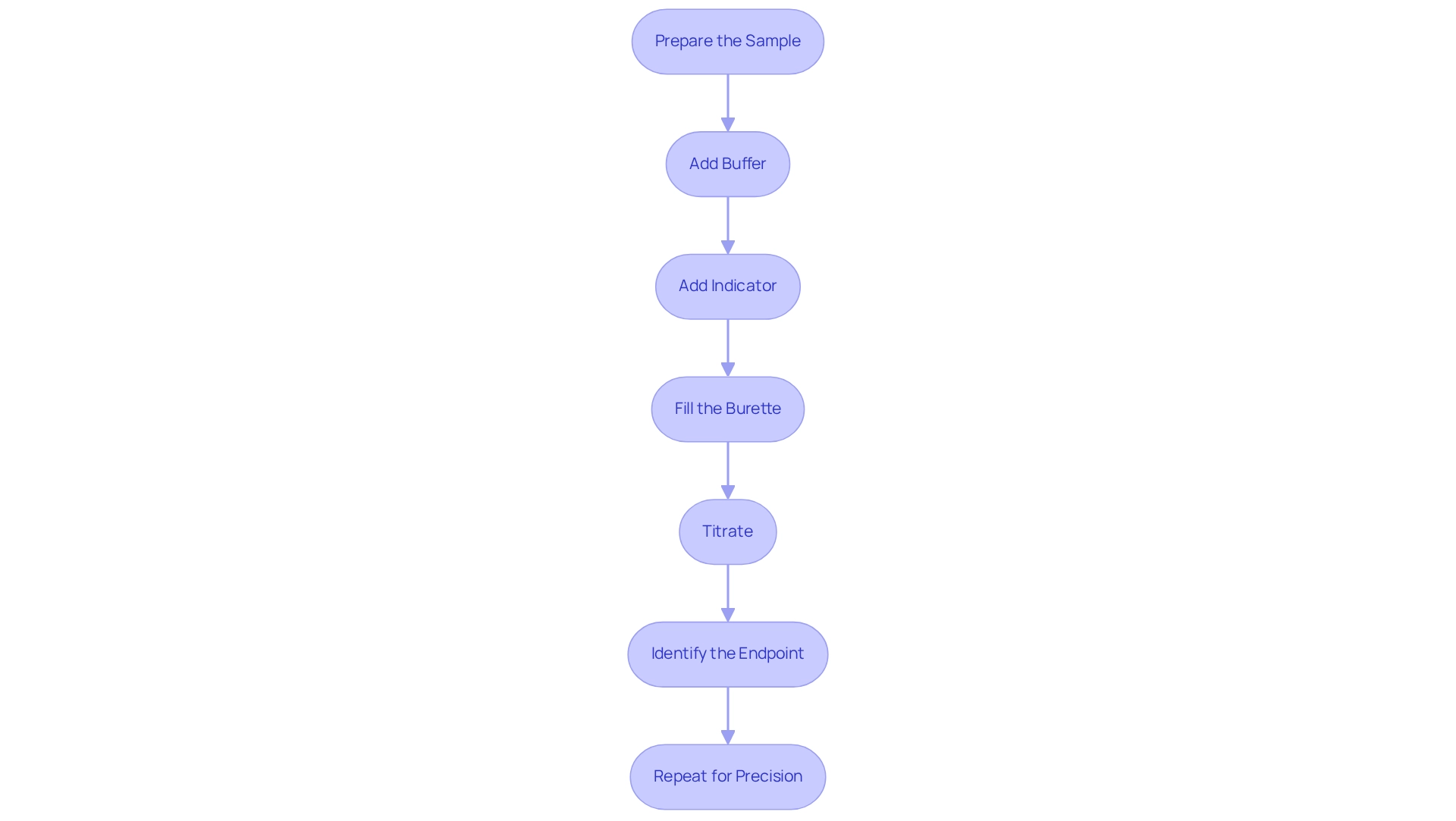
Follow the Step-by-Step Titration Procedure
- Prepare the Sample: Accurately measure 50 mL of the sample liquid using a pipette and transfer it to a conical flask. This initial step is crucial for ensuring the integrity of your results.
- Add Buffer: Introduce 1-2 mL of pH 10 buffer liquid to the sample to ensure the pH remains stable throughout the titration process. Maintaining the correct pH is essential, as the proportion of the chelating agent and conditional formation constants can fluctuate considerably with pH levels, potentially impacting your findings.
- Add Indicator: Incorporate 1-2 drops of a suitable indicator, such as Eriochrome Black T, which will change the mixture's color to wine-red. This visual cue is vital for monitoring the EDTA titration's progress.
- Fill the Burette: Rinse the burette with the chelating agent, then fill it with the standardized chelating liquid, ensuring that no air bubbles are trapped inside. Proper preparation of your equipment is key to achieving accurate measurements.
- Titrate: Gradually add the EDTA liquid from the burette to the sample while stirring continuously. Observe the solution for a change in hue. Engaging in statistical comparisons during this process can enhance your understanding of the titration's accuracy and reliability. A recent study demonstrated that students improved their analytical skills through methodical practice, underscoring the value of diligence in laboratory work.
- Identify the Endpoint: The endpoint is indicated by a color shift from wine-red to clear blue (for Eriochrome Black T). Document the amount of the chelating agent dispensed at this point, as this data is essential for your analysis.
- To improve precision, perform the procedure at least three times and determine the average amount of the reagent used. This repetition helps mitigate any discrepancies and ensures reliable results. As Benjamin Franklin wisely stated, "An investment in knowledge pays the best interest," highlighting the importance of thorough practice and analysis in achieving successful outcomes in laboratory settings.
Integrating statistical analysis in measurement techniques can further enhance your approach. For instance, a recent study showed that students who participated in statistical comparisons of different methods, including the use of a specific chelating agent, significantly enhanced their grasp of both analytical techniques and data interpretation. This highlights the value of methodical practice in achieving successful outcomes in laboratory settings. Additionally, tools like JAMOVI can enhance the learning experience by allowing for easy generation of values and visualizations, further supporting accurate data analysis.
Troubleshoot Common Issues in EDTA Titration
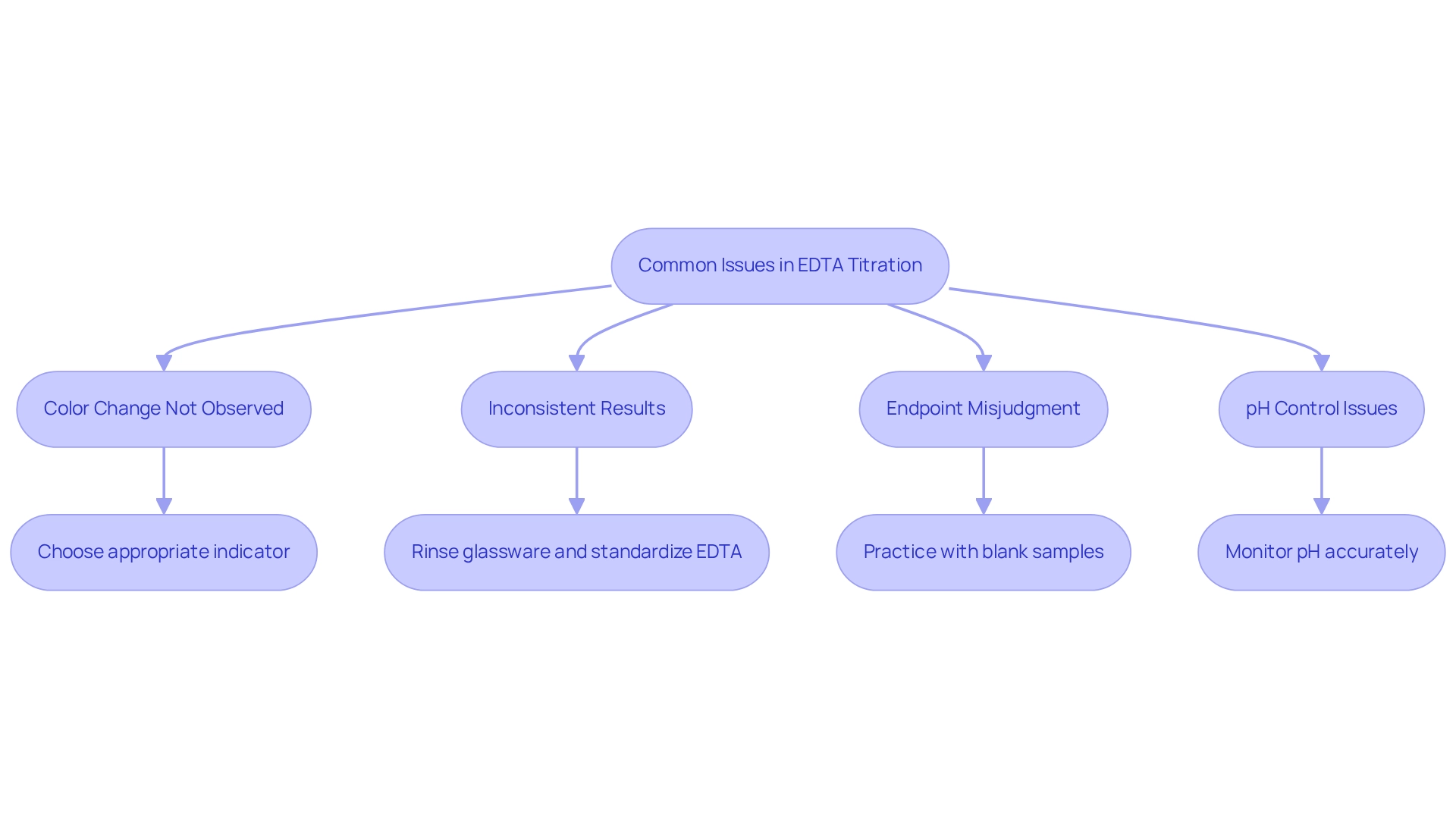
Common issues encountered during an EDTA titration can significantly impact the accuracy of results. Understanding these prevalent challenges and their solutions is crucial for achieving reliable outcomes:
- Color Change Not Observed: The choice of indicator is critical. Ensure that the indicator is appropriate for the specific metal ions being titrated. If the hue change remains unclear, consider switching to a more suitable indicator or adjusting the pH to enhance visibility. Data indicate that color change problems arise in approximately 30% of titration trials involving the chelating agent, underscoring the importance of selecting the right indicator.
- Inconsistent Results: Variability in results can often be traced back to contamination. Rinse all glassware with the substances being utilized to prevent cross-contamination. Additionally, ensure that the EDTA solution is properly standardized to maintain consistency across trials. Case studies demonstrate that improper reagent storage and environmental exposure can lead to significant inaccuracies, highlighting the necessity for chemists to exercise caution and vigilance in their methodologies.
- Endpoint Misjudgment: Misinterpreting the endpoint can lead to inaccuracies. To enhance your skills, practice noticing the hue change using a blank sample to familiarize yourself with the anticipated endpoint. Utilizing a white tile beneath the flask can also improve the visibility of the color change. Consistent practice is key to enhancing proficiency in identifying endpoints and executing measurements precisely, which is essential for overcoming this challenge.
- pH Control Issues: Maintaining the pH around 10 is vital for the stability of the metal-EDTA complex. Utilize a calibrated pH meter or pH strips to monitor and adjust the pH accurately throughout the neutralization process.
As Carl Sagan once said, "Somewhere, something incredible is waiting to be known." By acknowledging these frequent problems and applying the proposed remedies, you can greatly enhance the reliability and accuracy of your results in EDTA titration. Regular practice in determining endpoints and executing titrations accurately is paramount for overcoming these challenges.
Conclusion
Mastering EDTA titration is essential for obtaining reliable results in analytical chemistry. This article has delved into the foundational aspects of this technique, highlighting the critical nature of maintaining optimal pH levels, selecting suitable indicators, and implementing systematic troubleshooting methods. Each of these elements significantly contributes to ensuring that the titration process is both accurate and efficient.
A thorough understanding of the fundamentals of EDTA titration is vital for every chemist, regardless of their experience level. By utilizing standardized materials and adhering to a comprehensive, step-by-step procedure, practitioners can enhance their analytical skills and reduce the likelihood of errors. Furthermore, being cognizant of common challenges and their solutions enables better management of potential pitfalls during experimentation.
In summary, the importance of precision in EDTA titration is paramount. As this technique remains a cornerstone of analytical chemistry, the dedication to mastering its intricacies not only elevates individual competencies but also enriches the broader field of chemical analysis. By prioritizing meticulous methodology and a commitment to continuous learning, chemists can ensure that their results are both trustworthy and impactful.




