Overview
This article elucidates the principles, setup, and applications of Size-Exclusion Chromatography (SEC) within High-Performance Liquid Chromatography (HPLC). SEC is pivotal for separating molecules based on size, a process integral to the analysis of proteins and polymers. Particularly in the biopharmaceutical sector, SEC plays a crucial role in determining molecular weight and purity. This ensures product safety and efficacy, reinforcing the necessity of high-quality scientific instruments in laboratory settings. Understanding SEC's applications not only highlights its importance but also prompts further exploration of its capabilities in enhancing analytical precision.
Introduction
Size-Exclusion Chromatography (SEC) serves as a fundamental pillar in High-Performance Liquid Chromatography (HPLC), providing an advanced technique for the separation of molecules based on size. This method significantly improves the analysis of essential biopharmaceuticals, including monoclonal antibodies, while effectively addressing the challenges associated with high-molecular-weight species. As the biopharmaceutical industry experiences an increasing demand for precise analytical methods, one critical question arises: how can a thorough understanding of SEC's principles and applications reshape pharmaceutical analysis and uphold the quality of life-saving therapeutics?
Define Size-Exclusion Chromatography (SEC) in HPLC
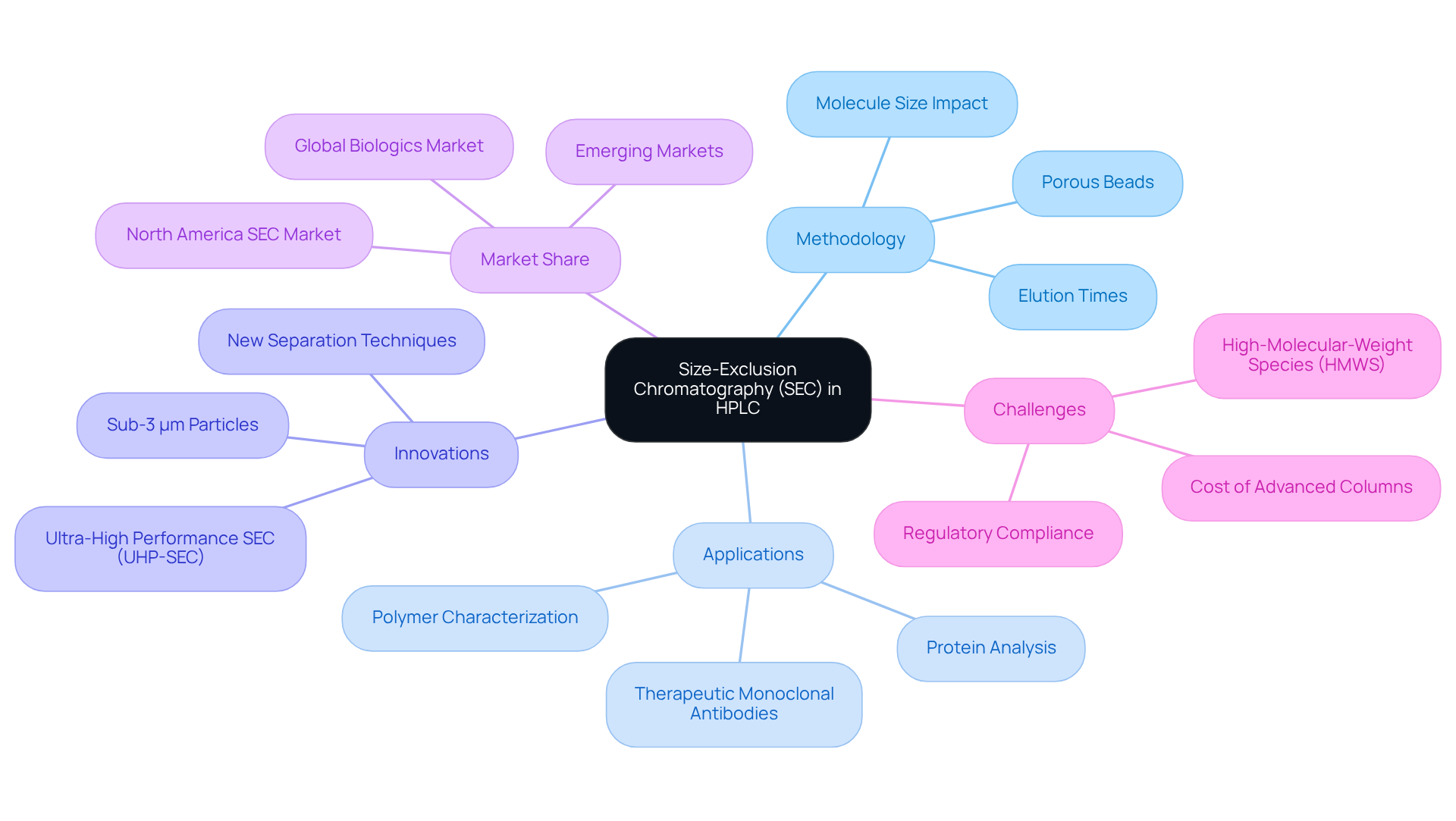
Size-Exclusion Chromatography (SEC) is a pivotal method utilized in HPLC SEC, adept at separating particles based on their dimensions. In this methodology, a sample is introduced into a tube filled with porous beads, where the size of the pores dictates the accessibility for different substances. Larger molecules are unable to traverse the pores, resulting in a quicker elution, while smaller molecules can penetrate the pores, thus experiencing a prolonged elution time as they navigate through the medium.
This technique proves particularly beneficial for analyzing proteins, polymers, and other macromolecules, providing essential insights into their size distribution and molecular weight. Recent innovations in SEC, notably the advent of ultra-high performance (UHP)-SEC setups employing sub-3 μm particles, have dramatically enhanced method throughput by 3- to 5-fold compared to conventional SEC. Such advancements are critical for characterizing therapeutic monoclonal antibodies (mAbs) and other biologics, meeting the escalating demand for precise analytical techniques in the biopharmaceutical sector.
Importantly, biologics represent over 25% of all pharmaceutical sales, with the global biologics market anticipated to surpass $750 billion by 2030. Furthermore, North America commands a 33% share of the global HPLC SEC market, highlighting the significance of HPLC SEC in this burgeoning field. The challenges posed by high-molecular-weight species (HMWS) in mAbs are recognized as critical quality attributes (CQAs), which SEC effectively addresses, thus ensuring the efficacy and safety of these therapeutic products.
Explain the Principles of SEC and Molecular Separation
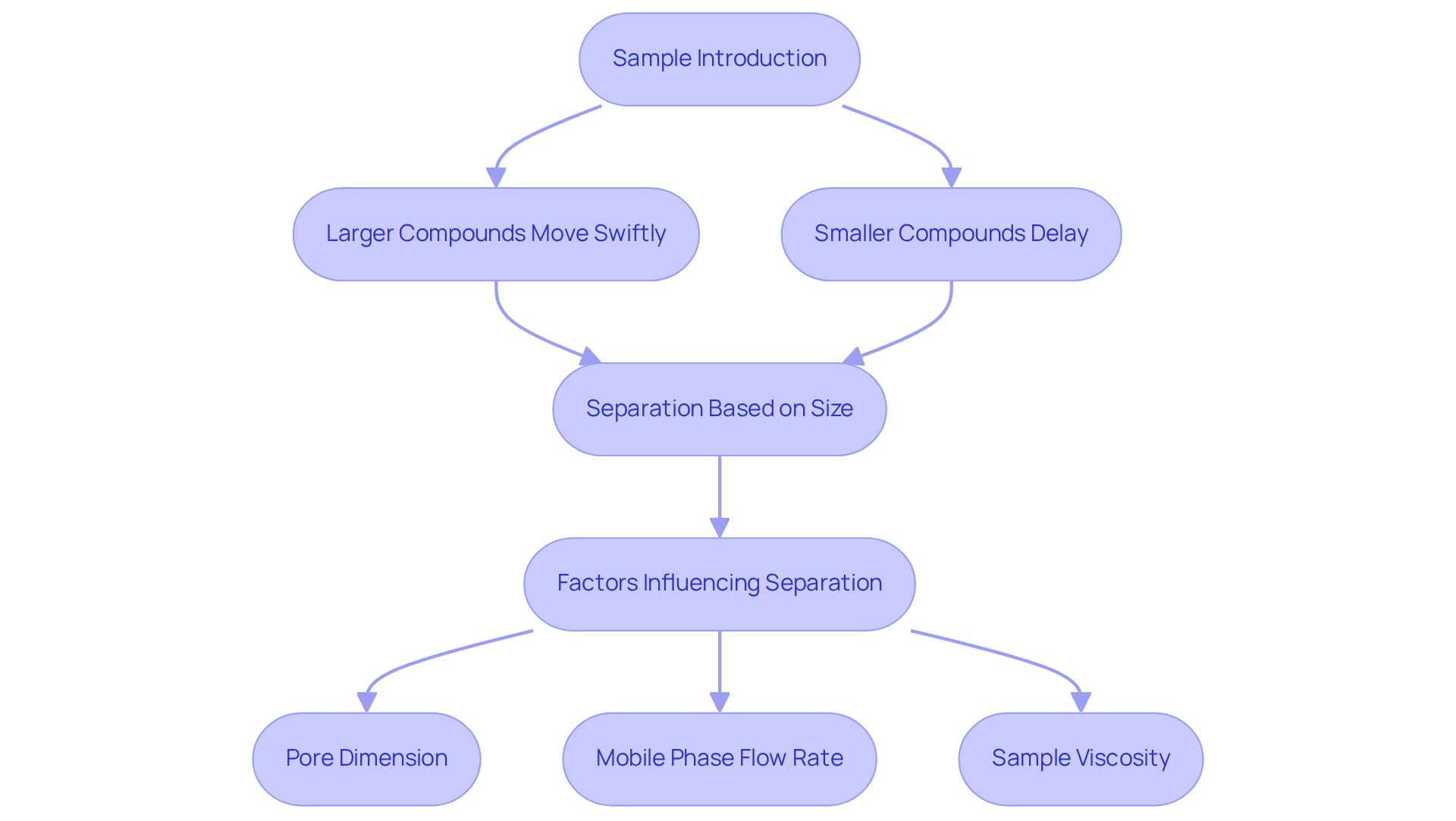
Size-Exclusion Chromatography (SEC), also known as hplc sec, operates on the principle of differential migration of particles through a porous medium, making it a pivotal technique in analytical chemistry. When a sample is introduced into the column, particles are separated according to their hydrodynamic volume.
- Larger compounds are unable to penetrate the stationary phase's pores, enabling them to move through the column more swiftly.
- Smaller substances can access these pores, leading to a delay in their passage.
This separation process in hplc sec is influenced by several critical factors, such as pore dimension, mobile phase flow rate, and sample viscosity. To optimize separation efficiency, the ideal pore dimension should be approximately three times the diameter of the target molecules.
For instance, the TSKgel SuperMultiporeHZ-M column features an average pore dimension of about 140 Å and accommodates a linear molecular weight range from 400 to 4,000,000. Consequently, HPLC SEC effectively categorizes samples into distinct range classifications, facilitating the analysis of molecular weight distributions and the identification of aggregates or fragments.
As Yuliia Orlova aptly notes, "Separating particles by their dimensions, exclusion chromatography (SEC) delivers the molecular weight distribution of the soluble elements of a polymer." This method is extensively utilized across various fields, particularly in the purification of proteins and polymers, where the use of hplc sec for accurate molecular weight determination is crucial.
Furthermore, maintaining temperature control is essential for reproducibility in SEC, as any fluctuations can significantly impact the results.
Discuss Applications of SEC in Pharmaceutical Analysis
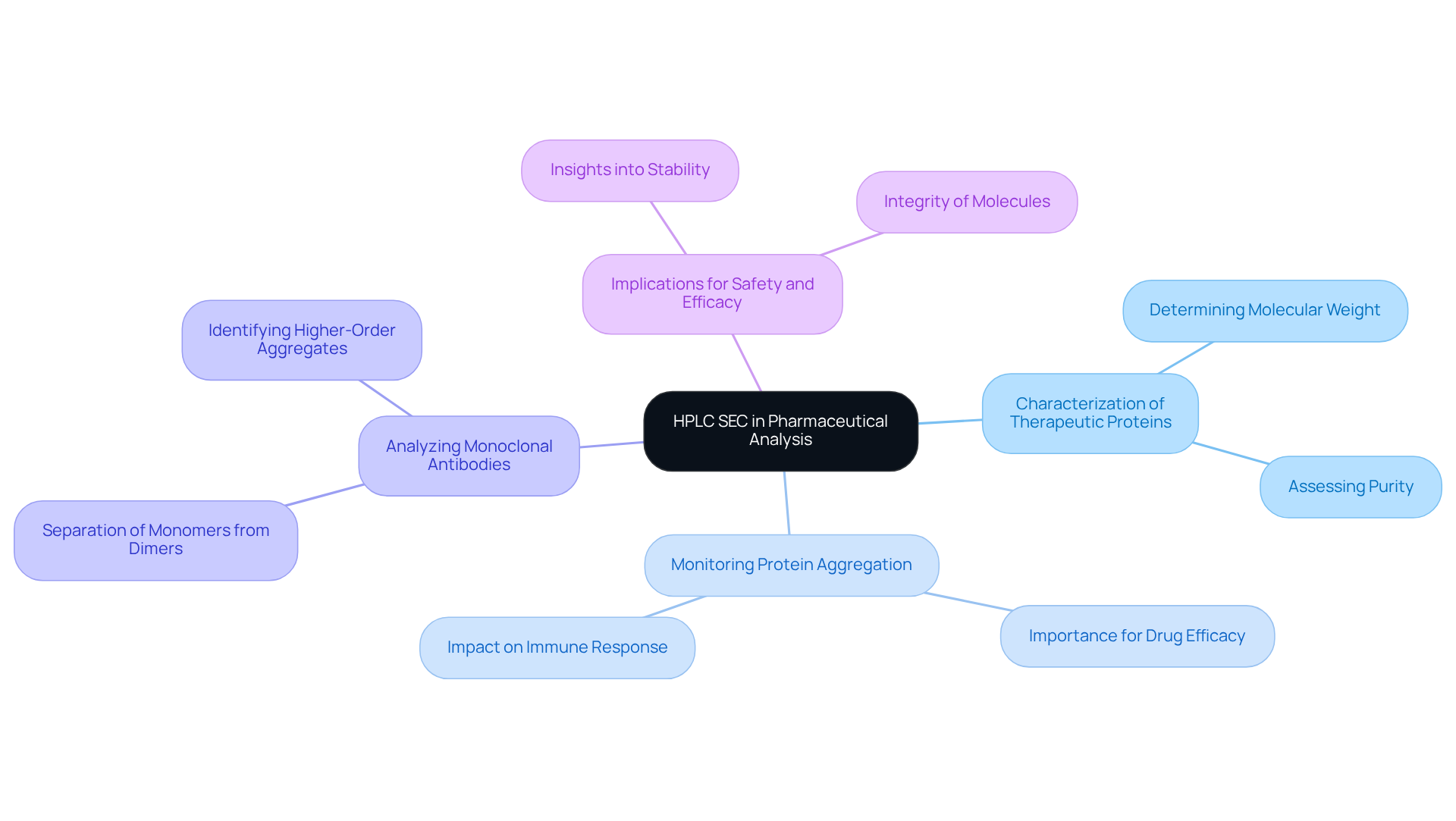
HPLC SEC plays a pivotal role in pharmaceutical analysis, serving several critical applications. Primarily, HPLC SEC is utilized in the characterization of therapeutic proteins, where it effectively determines the molecular weight and purity of protein formulations. This process is essential for monitoring protein aggregation, a vital quality attribute in biopharmaceuticals. Furthermore, HPLC SEC is instrumental in analyzing monoclonal antibodies, as it facilitates the separation of monomers from dimers and higher-order aggregates. Such analysis is crucial for ensuring the safety and efficacy of biopharmaceutical products, as it provides valuable insights into the stability and integrity of the molecules involved.
Outline Technical Setup and Sample Preparation for SEC
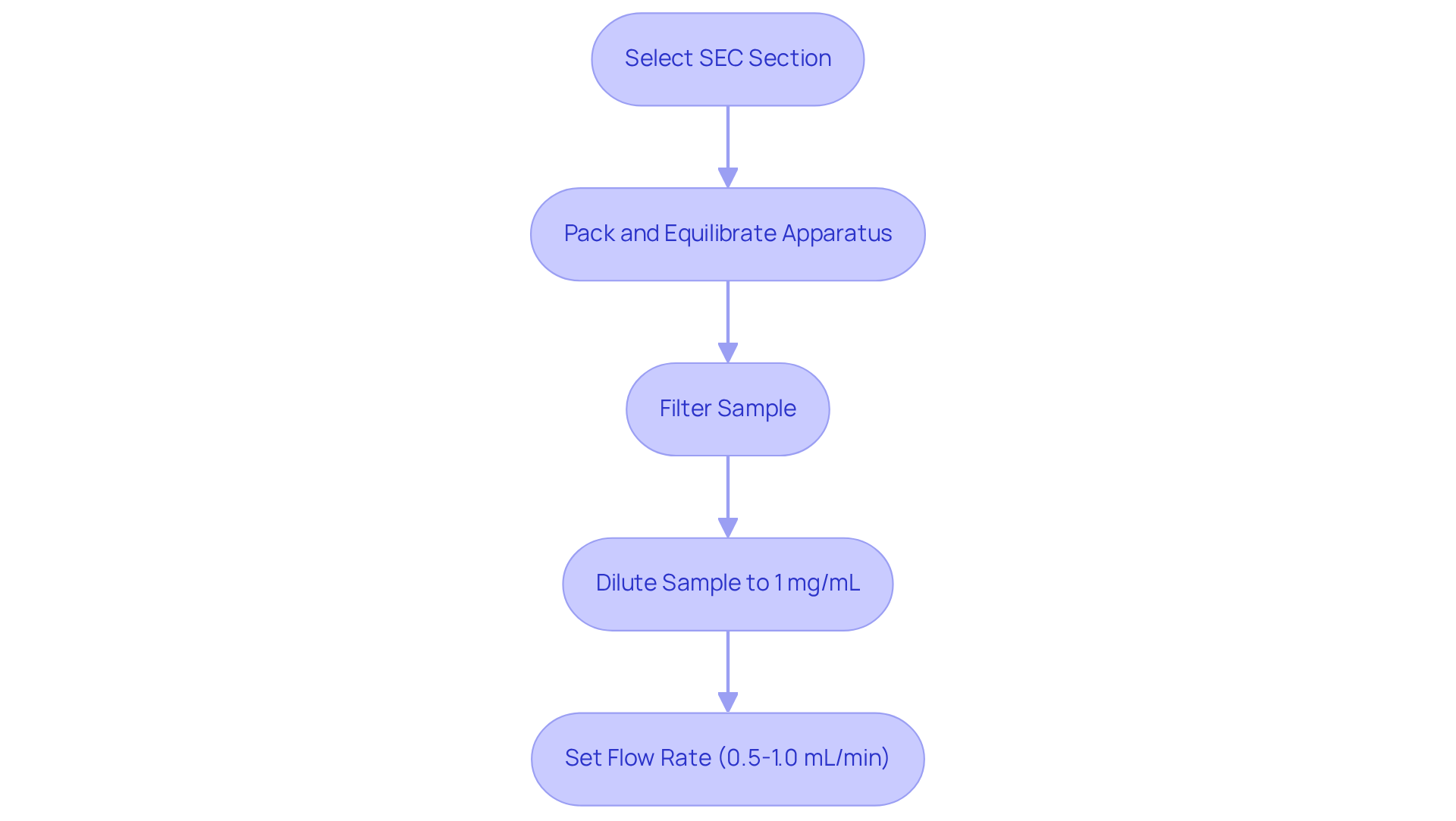
The successful execution of HPLC SEC (Size-Exclusion Chromatography) is fundamentally dependent on meticulous technical setup and sample preparation. To begin, it is essential to select an appropriate SEC section tailored to the molecular size range of the analytes. Proper packing and equilibration of the apparatus with a mobile phase—typically a buffer solution—are crucial for maintaining sample stability. Furthermore, sample preparation must involve filtering through a 0.22 or 0.45-micron filter to eliminate particulates that could obstruct the system. Diluting the sample to an optimal concentration, generally around 1 mg/mL, is vital to prevent excessive loading.
Setting the flow rate according to the manufacturer's specifications, which usually range from 0.5 to 1.0 mL/min, is essential for achieving effective separation and resolution. Real-world applications illustrate that strict adherence to these preparation techniques can significantly enhance the reliability of results obtained from HPLC SEC, particularly in the characterization of complex macromolecules such as proteins and polymers. For instance, employing size-exclusion columns like the Superdex 16/600, with a working range of approximately 10-600 kDa, can yield precise molecular weight determinations when combined with light scattering detection methods.
By following these guidelines, laboratories can optimize their HPLC SEC processes, ensuring high-quality analytical outcomes. This structured approach not only bolsters the accuracy of molecular characterizations but also reinforces the importance of rigorous sample preparation in achieving reliable results.
Conclusion
Size-Exclusion Chromatography (SEC) is an essential technique within High-Performance Liquid Chromatography (HPLC), facilitating the precise separation of molecules based on size. This method is critical across various domains, particularly in the biopharmaceutical industry, where it is integral to the characterization of proteins and the assurance of therapeutic product quality. By employing porous beads, SEC effectively differentiates larger molecules, which elute more rapidly, from smaller ones that traverse through the pores, thus yielding vital insights into molecular weight and distribution.
The principles of SEC are explored in detail, emphasizing the significance of parameters such as pore dimensions and flow rates in achieving optimal separation. The method's applications in pharmaceutical analysis are highlighted, particularly in monitoring protein aggregation and characterizing monoclonal antibodies—key factors in maintaining drug efficacy and safety. Furthermore, the technical setup and thorough sample preparation are underscored as crucial elements that substantially affect the reliability of results derived from HPLC SEC.
In summary, mastering HPLC SEC not only enhances analytical capabilities but also underscores the necessity of precision in pharmaceutical development. With the increasing demand for biologics, a comprehensive understanding and implementation of effective SEC techniques is becoming ever more critical. Laboratories are urged to adopt best practices in setup and sample preparation to ensure accurate and reliable outcomes, ultimately fostering the advancement of biopharmaceutical innovations.




