Overview
This article delves into mastering ion exchange column chromatography to achieve optimal results. By grasping its fundamental principles, components, and optimization techniques, readers can significantly enhance their chromatography practices.
Selecting appropriate materials and parameters is crucial; implementing advanced techniques such as gradient elution and temperature control further refines the process. Additionally, troubleshooting common issues is essential for improving both the efficiency and reliability of chromatography.
Ultimately, this comprehensive understanding not only elevates laboratory practices but also establishes a foundation for ongoing success in scientific endeavors.
Introduction
Ion exchange column chromatography is a cornerstone technique in biomolecule separation, leveraging the intricate balance of charge interactions to unravel the complexities of various mixtures. By mastering this method, scientists can significantly enhance the purity and efficiency of their analyses, whether in protein purification within biochemistry or in drug formulation in pharmaceuticals.
However, achieving optimal results presents its own set of challenges. How can one effectively navigate the intricacies of column selection, parameter optimization, and troubleshooting common pitfalls? This guide provides essential strategies and insights designed to elevate ion exchange chromatography practices to new heights.
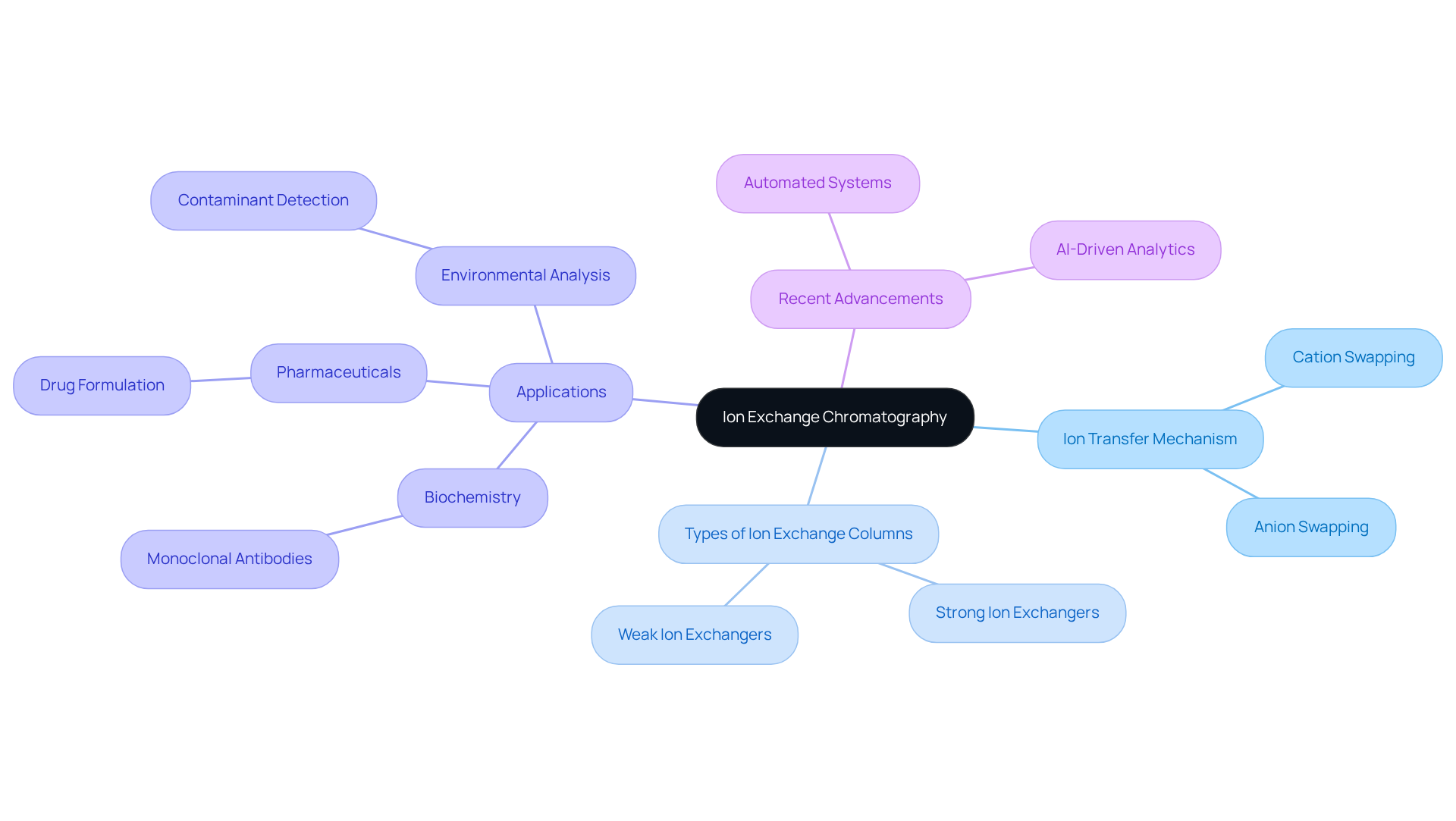
Understand the Basics of Ion Exchange Chromatography
Ion displacement separation represents a sophisticated method employed for the isolation and refinement of biomolecules based on their charge properties. This technique is based on ion exchange column chromatography, which relies on reversible interactions between charged molecules in a sample and the charged groups present on the stationary phase of the chromatography column. To effectively master this technique, it is essential to grasp the following key concepts:
-
Ion Transfer Mechanism: It is crucial to familiarize yourself with the principles of cation and anion transfer. In cation swapping, positively charged ions in the solution are substituted for other cations attached to the stationary phase, while anion swapping involves the replacement of negatively charged ions.
-
Types of Ion Exchange Columns: Differentiate between strong and weak ion exchangers, each tailored for specific applications. Strong ion exchange column chromatography operates effectively across a wide pH range, whereas weak ion exchange column chromatography is more selective and can be utilized under specific conditions.
-
Applications of ion exchange column chromatography find extensive use across various fields, including biochemistry for protein purification, pharmaceuticals for drug formulation, and environmental analysis for detecting contaminants. For instance, in biochemistry, this technique is crucial for isolating monoclonal antibodies, which are increasingly significant in therapeutic applications.
Recent advancements in ion separation techniques, such as the integration of automated systems and AI-driven analytics, are enhancing efficiency and precision within laboratory environments. These innovations are pivotal in meeting the growing demands for high-quality analytical methods in the pharmaceutical industry, particularly as the focus shifts towards personalized medicine and biologics.
By mastering these foundational concepts, you will be well-equipped to navigate the complexities of ion separation systems and leverage their capabilities for optimal results in your scientific endeavors.
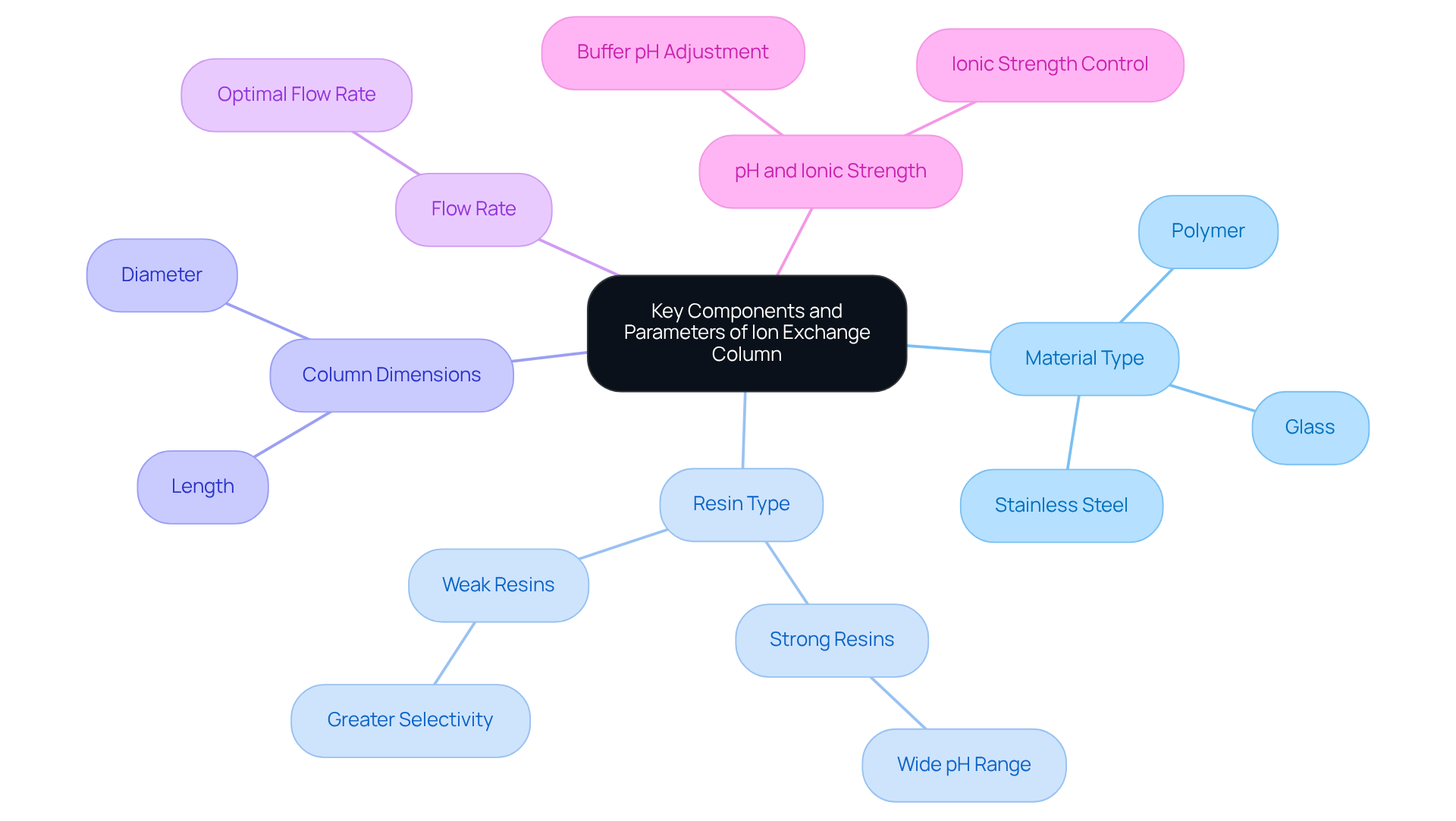
Identify Key Components and Parameters of Your Column
To achieve optimal results in ion exchange column chromatography, it is essential to identify and understand the key components and parameters of your column.
-
Material Type: Choose from glass, stainless steel, or polymer structures based on your application requirements and chemical compatibility.
-
Resin Type: Select the appropriate resin—strong or weak—according to the nature of the ions you wish to separate. Strong resins accommodate a wide pH range, while weak resins offer greater selectivity.
-
Column Dimensions: Evaluate the length and diameter of the column, as these dimensions significantly influence the resolution and capacity of your separation.
-
Flow Rate: Establish the optimal flow rate for your application, as this parameter affects the interaction time between the sample and the resin.
-
pH and Ionic Strength: Adjust the pH and ionic strength of your buffer to enhance the binding and elution of target molecules.
By thoughtfully selecting these elements and parameters, you can significantly enhance the efficiency and effectiveness of your ion exchange column chromatography technique.
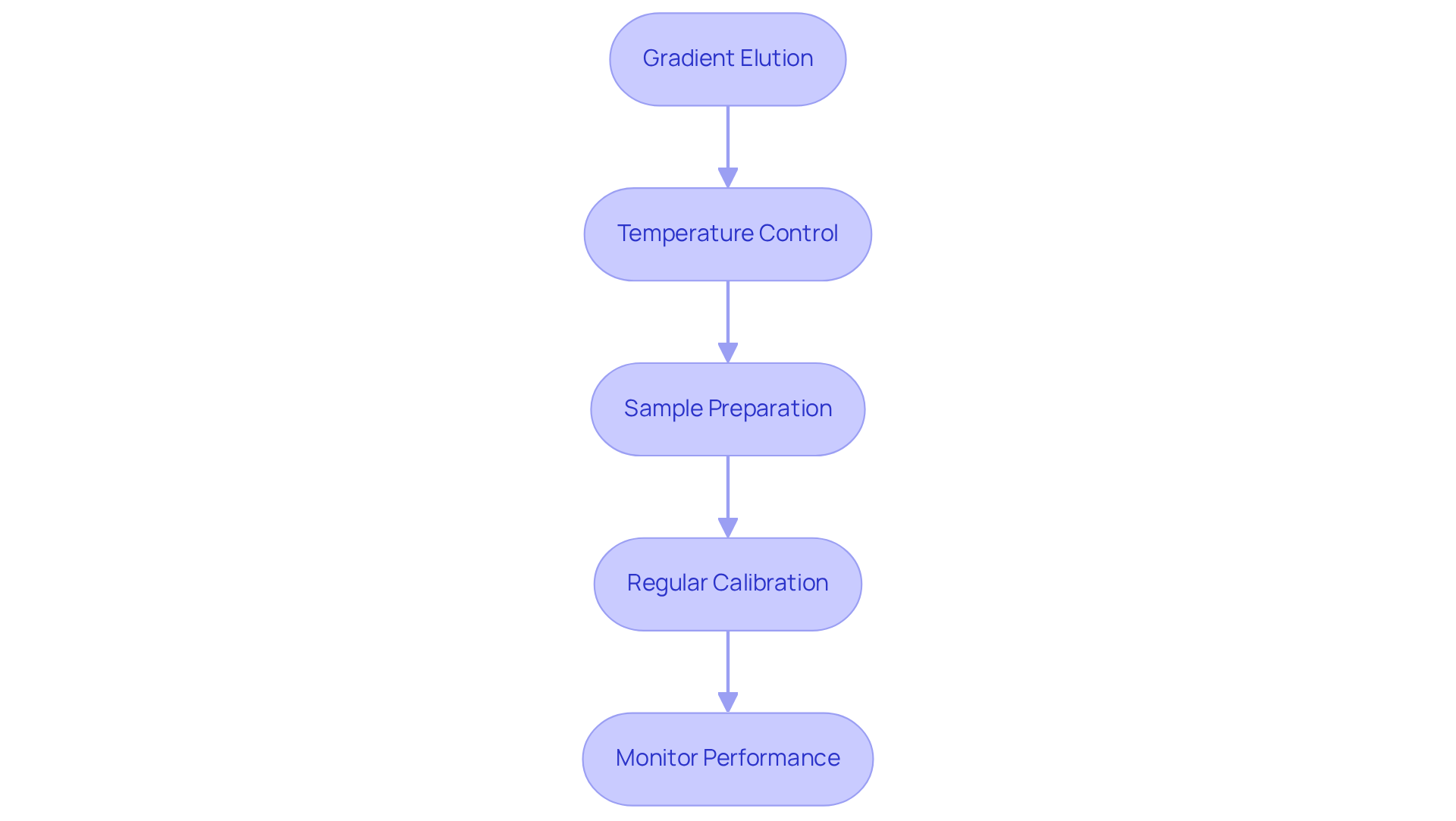
Implement Optimization Techniques for Enhanced Performance
To optimize your ion exchange chromatography process, consider implementing the following techniques:
-
Gradient Elution: Employ a gradient of increasing ionic strength to enhance the separation of closely related ions, significantly improving resolution. Recent studies have demonstrated that gradient elution not only shortens run times but also allows for higher sample loads while maintaining good resolution, making it a preferred method for complex mixtures. Notably, gradient columns have shown a ∼24% advantage in resolution compared to uniform columns, confirming their effectiveness in enhancing separation efficiency.
-
Temperature Control: Maintaining a consistent temperature throughout the separation process is crucial, as fluctuations can adversely affect ion interactions and separation efficiency. Studies suggest that accurate temperature management can result in enhanced reproducibility and clarity in ion separation techniques, ensuring optimal performance. Specifically, temperature stability is linked to enhanced ion interaction consistency, which is vital for achieving reliable results.
-
Sample Preparation: Proper sample preparation is essential. Ensure that samples are filtered and concentrated to minimize impurities that could interfere with separation. This step is essential for obtaining dependable results, as impurities can greatly affect the efficacy of the analysis system.
-
Regular Calibration: Regular calibration of your equipment is necessary to ensure accurate measurements and consistent performance. This practice helps in maintaining the integrity of the results and allows for timely adjustments to the system as needed.
-
Monitor Performance: Continuously track key performance indicators such as retention time, peak shape, and resolution. Monitoring these metrics enables you to identify areas for improvement and make informed adjustments to your methodology.
By utilizing these optimization methods, you can significantly improve the performance of your ion separation process with ion exchange column chromatography, leading to more dependable and consistent outcomes.
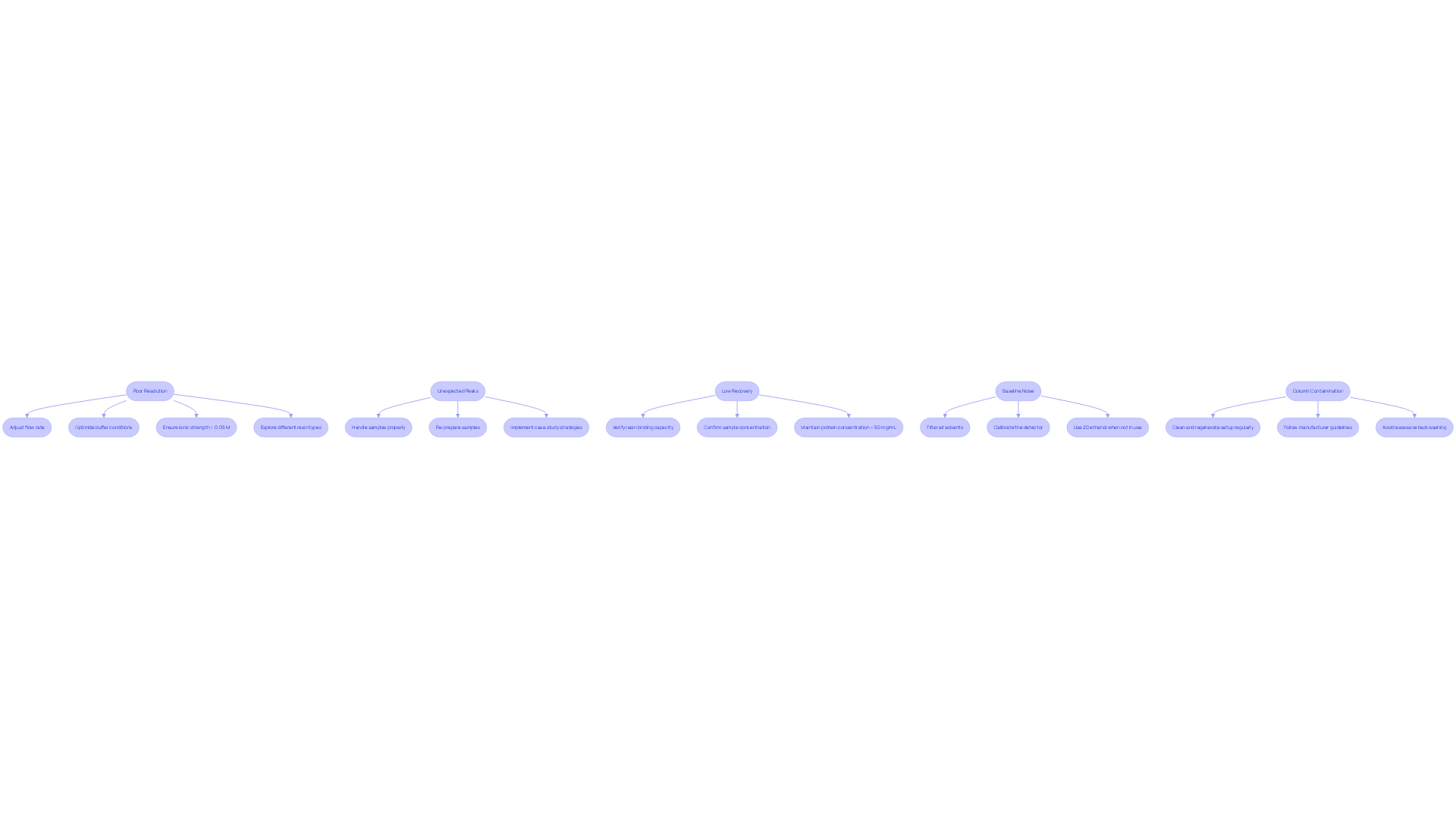
Troubleshoot Common Issues in Ion Exchange Chromatography
When working with ion exchange column chromatography, several common issues may arise that require attention. To effectively address these challenges, consider the following troubleshooting tips:
-
Poor Resolution: Should you encounter poor resolution, it is advisable to adjust the flow rate, optimize buffer conditions, and ensure that the ionic strength of buffers remains above 0.05 M. Additionally, exploring different resin types may yield improved performance, as both cation and anion resins can show varying results based on the specific application.
-
Unexpected Peaks: Unexpected peaks in your chromatogram may signify contamination or sample degradation. It is essential to handle samples properly and consider re-preparing them. Implementing strategies derived from case studies focused on overcoming sample loss and contamination can further enhance your results.
-
Low Recovery: In cases where recovery rates are low, verify the binding capacity of your resin and confirm that your sample concentration aligns with the specifications of the apparatus. Maintaining protein concentrations below 50 mg/mL is crucial for optimal binding.
-
Baseline Noise: High baseline noise may stem from impurities in the mobile phase or issues with the detector. Ensure that all solvents are filtered and that the detector is calibrated accurately. Utilizing 20% ethanol when the apparatus is not in use can also help prevent microbial growth, which contributes to baseline noise.
-
Column Contamination: It is vital to clean and regenerate your setup regularly to prevent fouling, which can lead to diminished performance over time. Adhering to manufacturer guidelines regarding regenerant concentration and application time is essential to avoid complications during resin regeneration. Furthermore, be mindful that excessive backwashing and mechanical failures can result in resin loss, adversely affecting column performance.
By implementing these troubleshooting steps and integrating additional strategies, you can effectively tackle common issues and sustain the performance of your ion exchange column chromatography.
Conclusion
Mastering ion exchange column chromatography is indispensable for those aiming to achieve optimal results in the separation and purification of biomolecules. This technique, rooted in the principles of ion displacement separation, facilitates the effective isolation of compounds according to their charge properties. A comprehensive understanding of its foundational concepts, from the ion transfer mechanism to the various types of columns and their diverse applications, is essential for fully harnessing its potential.
Key components, including the selection of material and resin type, alongside parameters such as column dimensions, flow rate, and buffer conditions, significantly influence the success of ion exchange chromatography. Implementing optimization techniques—such as gradient elution and temperature control—while maintaining vigilant monitoring and regular calibration can markedly enhance the performance of this method. Furthermore, equipping oneself to troubleshoot common issues is vital for ensuring consistent and reliable outcomes, thereby underscoring the necessity of mastering this technique.
The ongoing advancements in ion exchange chromatography, encompassing innovative technologies and automated systems, highlight its relevance across various fields, from biochemistry to pharmaceuticals. By embracing these practices and innovations, professionals can not only elevate their analytical capabilities but also contribute meaningfully to the evolving landscape of personalized medicine and biologics. The journey to mastering ion exchange chromatography transcends mere technique; it embodies the pursuit of advancing scientific understanding and achieving excellence in research and application.




