Overview
The article underscores the critical importance of mastering infrared (IR) spectrum ranges for precise chemical analysis. It highlights how different regions of the IR spectrum correspond to specific molecular vibrations, which are essential for the identification of various compounds. By detailing the distinct IR spectrum ranges, their associated molecular vibrations, and their applications in sectors such as pharmaceuticals and environmental monitoring, the article illustrates the vital role of accurate spectral analysis in both compound identification and quality assurance. This mastery not only enhances analytical capabilities but also ensures reliability in scientific research and industrial applications.
Introduction
In the realm of analytical chemistry, infrared spectroscopy emerges as an essential instrument for the identification and analysis of chemical substances. By leveraging the unique interactions between infrared radiation and molecular structures, this technique unveils the intricate vibrational motions of chemical bonds, yielding a distinctive spectral fingerprint for each sample.
As technological advancements propel its applications across diverse fields—from environmental monitoring to pharmaceutical quality control—the importance of mastering infrared spectroscopy has reached unprecedented heights.
This article explores the foundational principles of IR spectroscopy, examines the critical regions of the IR spectrum, and highlights its practical applications in both research and industry, demonstrating how this powerful technique continues to evolve and shape modern science.
Explore the Basics of Infrared Spectroscopy
Infrared analysis (IR) serves as a pivotal analytical method for identifying and examining substances through their interaction with radiant heat. When radiant energy in the infrared spectrum is directed at a sample, specific wavelengths are absorbed by the molecules, inducing vibrational movements—either stretching or bending of molecular bonds. This interaction generates a spectrum that serves as a unique fingerprint for the sample, enabling chemists to identify functional groups and molecular structures, with the IR spectrum ranging from 4000 cm⁻¹ to 400 cm⁻¹, corresponding to the vibrational transitions of various substances. Mastery of these fundamental principles is essential for the accurate interpretation of IR spectra.
Recent advancements in thermal analysis have significantly enhanced its applications in chemical examination. The market increasingly recognizes the importance of infrared analysis in environmental monitoring, driven by growing concerns about air pollution and its health implications. This trend highlights the technique's versatility across multiple sectors, including pharmaceuticals and environmental science. The rising awareness of air pollution's impact on health is amplifying the demand for IR analysis in environmental contexts. Moreover, case studies illustrate the practical applications of this technology. For instance, Bruker Corporation's acquisition of Canopy Biosciences in September 2020 expanded its capabilities in biomarker imaging, exemplifying the integration of IR techniques in advanced life science research and diagnostics. This acquisition fortifies Bruker's position in the market by enhancing its technological capabilities in thermal imaging.
As we approach 2025, the thermal imaging market continues to evolve, with advancements aimed at improving precision and effectiveness in material evaluation. Notably, MIR spectroscopy has demonstrated R² values ranging from 0.74 to 0.89 in predictive models for bioactive antioxidants in strawberry juice fermentation, underscoring its effectiveness in targeted applications. Understanding how infrared radiation interacts with substances not only enhances analytical accuracy but also facilitates the development of new methodologies in substance examination. Agilent Technologies has introduced the Cary 7000 FTIR spectrometer to meet the increasing demand for precision in material evaluation and quality control across diverse sectors, further emphasizing the importance of progress in this field.
Analyze IR Spectrum Ranges and Their Significance
The distinct regions of [[the IR spectrum ranges](https://blog.jmscience.com/comparing-ir-range-technologies-which-one-is-right-for-your-application)](https://blog.jmscience.com/comparing-ir-range-technologies-which-one-is-right-for-your-application) correspond to specific molecular vibrations that are critical for chemical analysis.
4000-3000 cm⁻¹: This region is primarily associated with C-H stretching vibrations, indicative of alkanes, alkenes, and aromatics. These vibrations are essential for identifying various organic compounds.
3000-2500 cm⁻¹: This range typically includes C-H stretches of terminal alkynes, providing insights into the presence of these functional groups in a sample.
2000-1500 cm⁻¹: Here, C≡C and C=O stretches can be observed, which are crucial for identifying alkynes and carbonyl compounds, respectively. The ability to detect these stretches aids in the characterization of complex molecules.
1500-1000 cm⁻¹: Known as the fingerprint region, this area contains intricate vibrations unique to specific molecules, making it invaluable for compound identification. The complexity of this region often requires careful interpretation, as variations in band intensities and solvent sensitivity can complicate results. Recent studies highlight the need for caution when interpreting results from this region, emphasizing the importance of cross-checking with other analytical methods to confirm findings.
Understanding the IR spectrum ranges is vital for chemists, as it allows them to deduce the presence of functional groups and assess the molecular structure of unknown compounds. For instance, the IR spectrum of hexanal reveals a C=O stretch peak just after 1700 cm⁻¹, underscoring the importance of precise spectral analysis in identifying chemical structures. Furthermore, merging thermal analysis with other analytical techniques, such as nuclear magnetic resonance and mass analysis, enhances the reliability of results. This multifaceted approach is crucial, particularly when analyzing outcomes from challenging areas of the spectrum, as suggested in recent research on infrared techniques. As Sagar aptly noted, "Best Review on IR," emphasizing the significance of thorough analysis in this field.
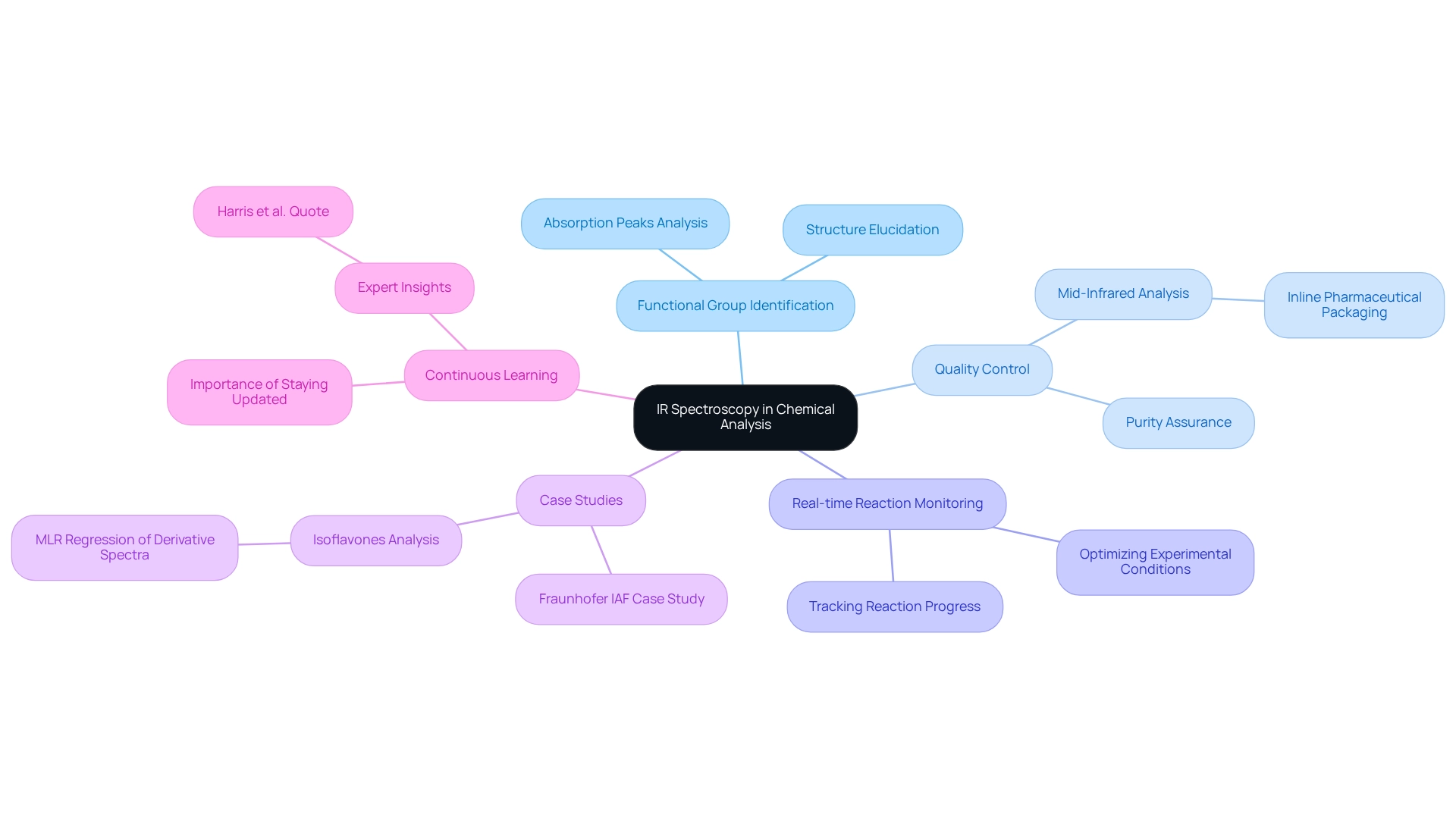
Apply IR Spectroscopy in Chemical Analysis and Research
IR spectroscopy plays a crucial role in both academic and industrial environments, particularly in pharmaceutical laboratories, where its applications are diverse and impactful. By examining the absorption peaks within the IR spectrum ranges, chemists can accurately identify functional groups within a compound, facilitating structure elucidation and enhancing the understanding of chemical behavior. In the pharmaceutical sector, IR analysis is essential for ensuring the purity of compounds and verifying the identity of both raw materials and finished products. This technique provides a reliable method for quality assurance, critical for compliance with industry standards. Researchers employ IR analysis to track changes in reaction mixtures in real-time, offering valuable insights into reaction progress and product formation. This capability allows for timely adjustments in experimental conditions, optimizing outcomes. When faced with unfamiliar substances, analyzing the IR spectrum ranges serves as a quick and efficient method for identifying chemical structures through their unique spectral fingerprints.
Real-world applications highlight the importance of infrared analysis in quality control. A case study from the Fraunhofer IAF illustrates the use of mid-infrared analysis with fast tunable quantum cascade lasers (QCLs) for inline quality control of pharmaceutical packaging. This implementation not only enhances measurement reliability but also streamlines quality assurance processes. The findings of this research have been published in the IEEE Sensors Journal, further emphasizing the significance of these advancements.
Moreover, the analysis of isoflavones in soybeans achieved through MLR regression of derivative spectra illustrates the effectiveness of IR spectroscopy in analyzing complex compounds, which is particularly relevant in pharmaceutical contexts. Expert insights emphasize the importance of continuous learning in analytical chemistry, as noted by Harris et al., who stated, "Continuous learning and exposure to the latest advancements are essential for practitioners in the fast-evolving field of analytical chemistry." By mastering these applications, chemists can significantly enhance their analytical capabilities, driving progress in research and product development.
Conclusion
The exploration of infrared spectroscopy underscores its essential role in the identification and analysis of chemical substances. By comprehending the intricate interactions between infrared radiation and molecular structures, chemists can derive unique spectral fingerprints that facilitate the identification of functional groups and molecular configurations. The segmentation of the IR spectrum into distinct regions further emphasizes the technique's precision, allowing for detailed analysis of various compounds.
Demonstrated through numerous applications in both academic and industrial settings, the relevance of infrared spectroscopy is paramount. From ensuring quality control in pharmaceuticals to monitoring reaction progress in real-time, this technique serves as a vital tool for chemists. The integration of advanced technologies, such as quantum cascade lasers, enhances the reliability and efficiency of infrared spectroscopy, paving the way for innovative applications across diverse fields.
Ultimately, mastering infrared spectroscopy not only elevates analytical capabilities but also supports ongoing advancements in chemical analysis. As the technique continues to evolve, it solidifies its position as an indispensable asset in the modern scientific landscape. Embracing these advancements empowers chemists to confront complex challenges and contribute to the evolving narrative of analytical chemistry, ensuring that the impact of infrared spectroscopy resonates well into the future.




