Overview
This article presents a detailed, step-by-step procedure for utilizing a Karl Fischer titrator, highlighting the significance of precise moisture analysis across various industries, especially pharmaceuticals. It begins by outlining the essential equipment and reagents required for the process. Following this, the article meticulously describes the titration process, ensuring clarity in each step. Troubleshooting tips are also provided, aimed at guaranteeing accurate results. This comprehensive approach underscores the method's vital role in maintaining product quality and ensuring compliance with regulatory standards, ultimately reinforcing the necessity of high-quality scientific instruments in laboratory settings.
Introduction
In the realm of analytical chemistry, few methods stand out as prominently as Karl Fischer titration, a technique revered for its precision in measuring moisture content across diverse substances. As industries such as pharmaceuticals, food, and petrochemicals increasingly prioritize product quality and stability, the significance of mastering this titration method cannot be overstated.
By delving into the underlying chemistry, essential equipment, and systematic procedures, professionals can harness the full potential of Karl Fischer titration. This article explores the intricacies of this method, from its foundational principles to troubleshooting common challenges, ultimately highlighting its indispensable role in maintaining the integrity of products in an ever-evolving market.
Understand the Basics of Karl Fischer Titration
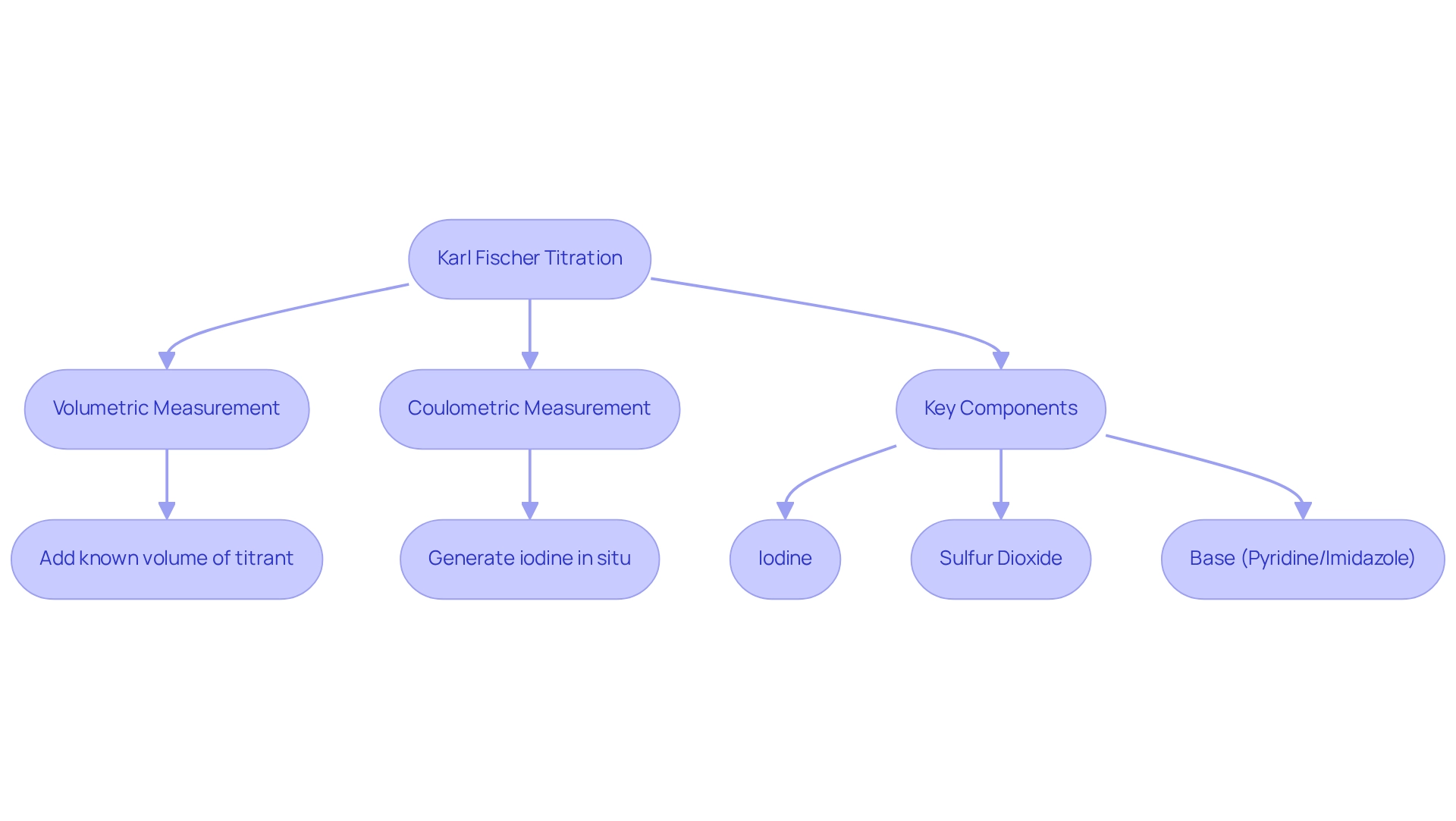
The method is a fundamental analytical technique employed to accurately assess moisture levels in various materials. This method is based on a redox reaction where iodine reacts with water, facilitated by a solvent—typically methanol—and a base. The process can be executed volumetrically or coulometrically, depending on the required precision and the properties of the sample. A solid understanding of the underlying chemistry, including the stoichiometry of the reaction, is essential for obtaining reliable results. This method is particularly vital in sectors such as pharmaceuticals, food, and petrochemicals, where precise moisture measurement is critical for ensuring product quality and stability.
Key components of this method include the primary reagents: iodine, sulfur dioxide, and a base (commonly pyridine or imidazole) dissolved in a solvent. Measurement types vary; volumetric measurement involves adding a known volume of titrant until the endpoint is reached, while coulometric measurement generates iodine in situ, making it suitable for measuring very low water content. This method plays a crucial role in quality control across various industries, ensuring compliance with regulatory standards and specifications, especially in pharmaceuticals, where the Hiranuma Aquacounter AQV-300 Volumetric and AQ-300 Coulometric Analyzers are utilized for moisture analysis in accordance with the Japanese Pharmacopoeia.
As we look ahead to 2025, the significance of moisture content analysis in pharmaceuticals continues to escalate, driven by the increasing demand for high-quality biotherapeutic products. Recent advancements in the Karl Fischer titrator method of moisture analysis have improved precision and effectiveness, making it a preferred option for pharmaceutical laboratories. Current statistics reveal a substantial rise in the adoption of this method, underscoring its relevance in maintaining product integrity and efficacy. Notably, the ideal pH range of the sample solution for this method is between 5.5 and 8, which is crucial for achieving optimal results. Real-world applications illustrate its effectiveness in establishing specifications based on historical manufacturing data, highlighting the integration of scientific understanding into the specification-setting process. As the pharmaceutical landscape evolves, this method remains a cornerstone for ensuring the quality and safety of pharmaceutical products.
Gather Necessary Equipment and Reagents
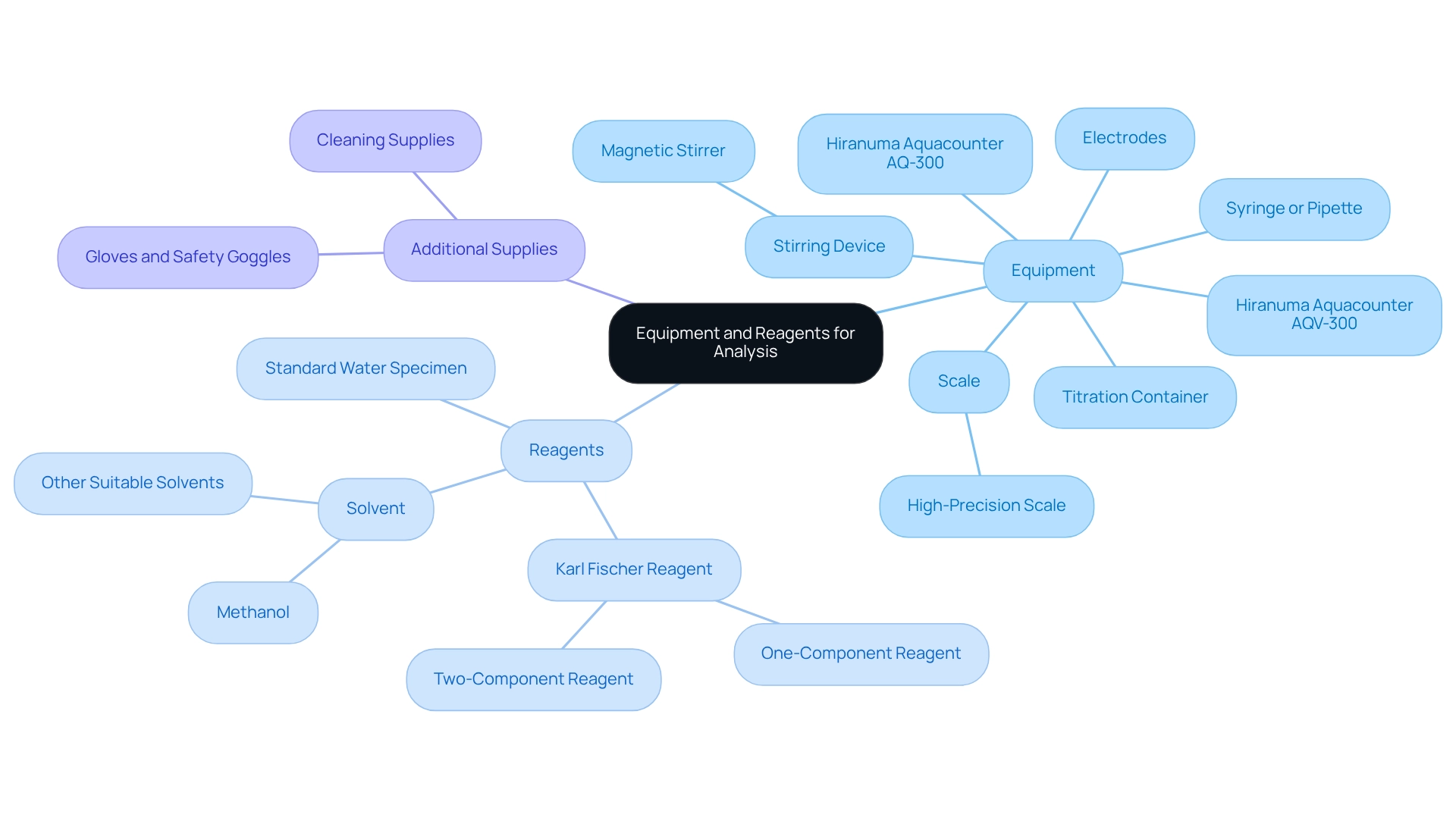
To effectively perform the analysis, it is essential to collect the suitable tools and substances:
Equipment:
- Choose between the Hiranuma Aquacounter AQV-300 volumetric titrator or the AQ-300 coulometric titrator for your Karl Fischer titrator needs. Both are designed for precise moisture analysis in drug and medicine testing using a Karl Fischer titrator, in compliance with the Japanese Pharmacopoeia. The AQV-300 is known for its volumetric accuracy, while the AQ-300 excels in coulometric precision, making them reliable choices for pharmaceutical applications when utilized with a Karl Fischer titrator.
- Titration Container: Utilize a clean, dry vessel that can hold both the specimen and the reagents.
- Syringe or Pipette: Employ a precise syringe or pipette for accurate introduction of the substance into the measuring container.
- Stirring Device: A magnetic stirrer is essential for ensuring thorough mixing of the mixture and reagents, which is critical for accurate results.
- Scale: Utilize a high-precision scale to accurately measure solid materials, as this directly influences the outcome of the analysis.
- Electrodes: Ensure you have the correct detector electrodes compatible with your titrator model to facilitate accurate measurements.
Reagents:
- Karl Fischer Reagent: Based on your method of analysis, select either a one-component or two-component reagent.
- Solvent: Typically, methanol or another suitable water-free solvent is used to dissolve the sample.
- Standard Water Specimen: A standard water specimen is necessary for calibration and validation of measurement outcomes, ensuring the accuracy of your readings.
Additional Supplies:
- Gloves and Safety Goggles: Always prioritize safety by wearing appropriate personal protective equipment when handling chemicals.
- Cleaning Supplies: Maintain a clean workspace by ensuring all glassware and equipment are free from contamination, which can affect results.
Practical Insights:
Different methods to release water from solid materials include dissolution in KF solvent, mechanical crushing, extraction with a suitable solvent, and evaporation in a dedicated KF oven. As the market for moisture measurement in the pharmaceutical industry is projected to grow significantly, selecting the right equipment and reagents becomes increasingly important. Expert recommendations suggest that lab managers should consider not only the specifications of the Hiranuma Aquacounter titrators but also the support and resources provided by manufacturers to ensure optimal performance.
Follow the Step-by-Step Titration Procedure
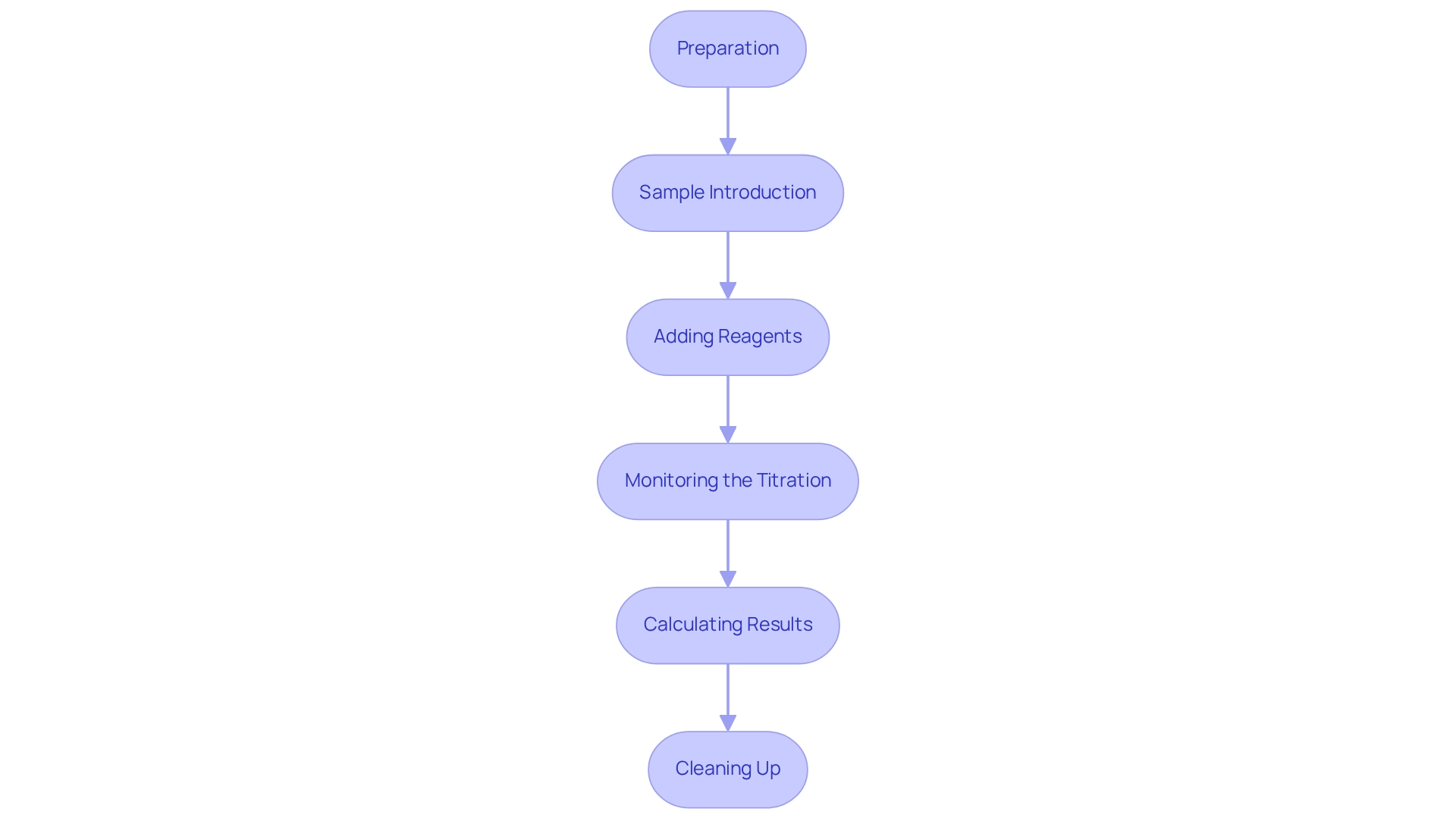
- Preparation: Begin by ensuring that all equipment is meticulously cleaned and dried. Rinse the measuring vessel with the solvent to eliminate any potential contaminants that could influence results. Proper cleaning of glassware is essential; immediate cleaning after use is crucial to prevent reagent spoilage. Calibrate the titrator according to the manufacturer's specifications to guarantee accurate measurements.
- Sample Introduction: Accurately weigh the sample using an analytical balance. For solid samples, ensure they are finely powdered to facilitate even moisture distribution. Introduce the sample into the titration vessel using a syringe or pipette, ensuring minimal exposure to ambient humidity, which can significantly impact the results.
- Adding Reagents: Carefully add the reagent for the Karl Fischer titrator to the titration vessel. If utilizing a volumetric titrator, set the initial volume precisely. Initiate the titration process, allowing the titrator to dispense the reagent until the endpoint is reached, which is critical for accurate moisture content analysis.
- Monitoring the Titration: Continuously observe the titration curve displayed on the instrument. The endpoint is typically marked by a sudden change in the measured parameter, such as potential or current. Record the volume of titrant used at the endpoint, as this data is essential for subsequent calculations.
- Calculating Results: Utilize the volume of titrant consumed to calculate the moisture content in the sample. Apply the appropriate formula, taking into account the titer of the reagent for accuracy.
- Cleaning Up: Post-titration, clean the titration vessel and electrodes following the manufacturer's guidelines. This step is crucial for maintaining the longevity and reliability of the equipment, ensuring it remains in optimal condition for future analyses, particularly when using the Karl Fischer titrator, which represents a significant advancement in the analytical field by providing reliable results for moisture analysis.
Troubleshoot Common Issues in Karl Fischer Titration
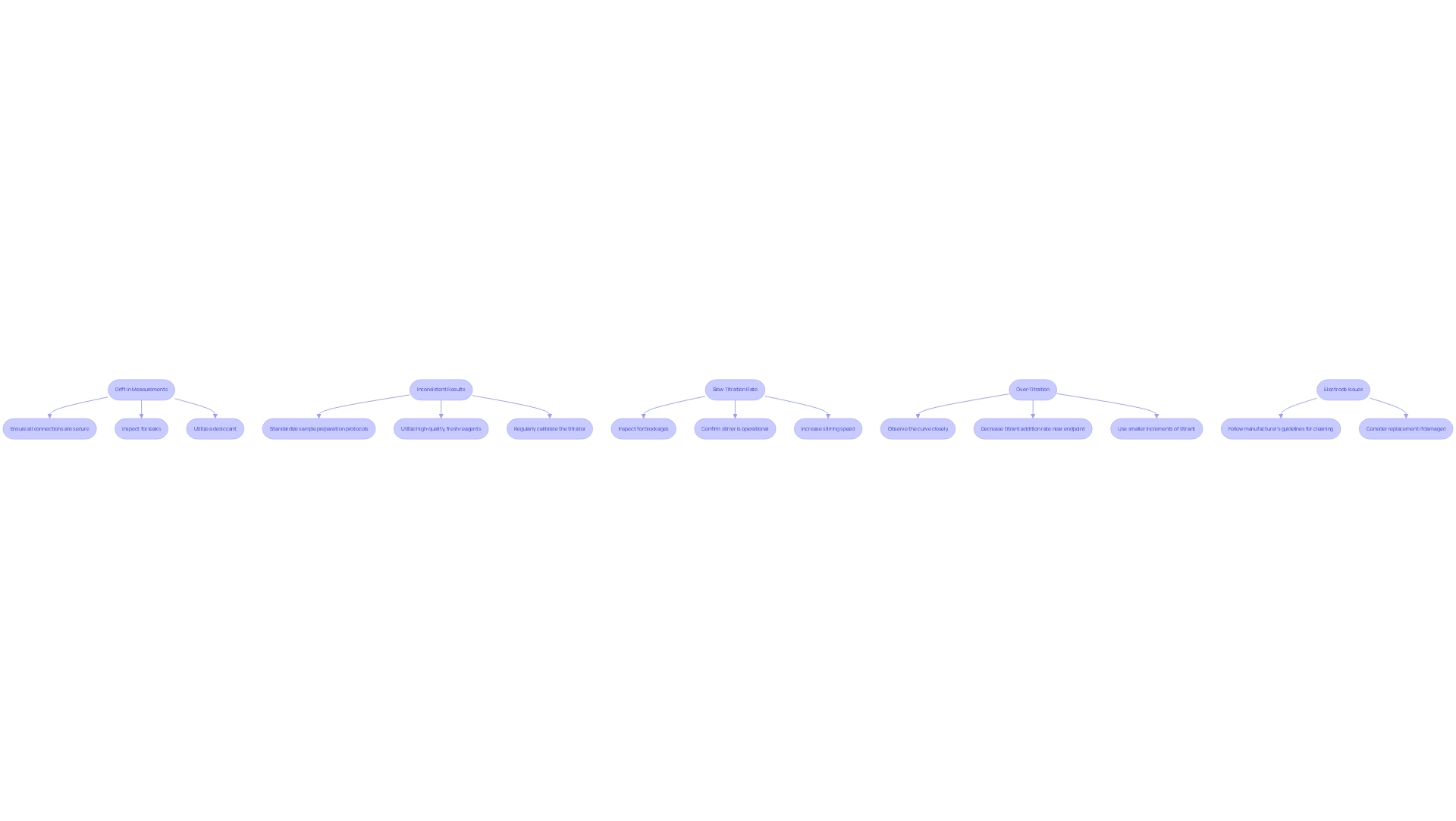
- Drift in Measurements: Atmospheric moisture infiltration or reagent contamination can lead to measurement drift. To address this, ensure all connections are secure and inspect for leaks. Utilizing a desiccant can help maintain a dry environment, crucial for accurate results.
- Inconsistent Results: Variability in sample preparation or subpar reagent quality may cause inconsistent results. Standardizing sample preparation protocols and utilizing high-quality, fresh reagents are essential steps. Regular calibration of the titrator is also vital. As Bettina Straub-Jubb states, "The foundation for every precise and dependable measurement method consists of high quality volumetric solutions and accurate high purity standards for a correct and reliable titer determination."
- Slow Titration Rate: Blockages in the titration cell or inadequate stirring can result in a slow titration rate. Inspect for any blockages and confirm that the stirrer is operational. If necessary, increase the stirring speed to enhance mixing and improve efficiency.
- Over-Titration: Adding excessive titrant beyond the endpoint leads to over-titration. It is crucial to closely observe the curve and decrease the titrant addition rate as the endpoint approaches. Employing smaller increments of titrant near the endpoint can significantly enhance accuracy.
- Electrode Issues: Contaminated or damaged electrodes can result in inaccurate readings. Following the manufacturer's guidelines for cleaning electrodes is essential, and replacement should be considered if any signs of damage are present.
In 2025, laboratories frequently encounter these issues, with reagent quality significantly affecting measurement results. Case studies highlight that the karl fischer titrator method is recognized as the gold standard for water content analysis across multiple industries, which reinforces the reliability of this technique. Furthermore, modern karl fischer titrators now include expiration date warnings, which aid users in planning for titrant replacement and minimizing downtime. By implementing these troubleshooting tips, laboratories can enhance accuracy and reliability in moisture analysis, solidifying the Karl Fischer titrator method as the benchmark across various sectors.
Conclusion
Mastering Karl Fischer titration is essential for professionals in industries where moisture content directly impacts product quality and stability. This method's reliance on a precise redox reaction enables accurate moisture measurement, rendering it invaluable in sectors such as pharmaceuticals, food, and petrochemicals. By understanding the foundational principles, gathering the appropriate equipment and reagents, and following systematic procedures, professionals can significantly enhance the reliability of moisture analysis.
Key components of the Karl Fischer titration process have been highlighted throughout this exploration. These include:
- The importance of high-quality reagents
- Meticulous sample preparation
- A robust troubleshooting framework
Addressing common issues such as measurement drift or inconsistent results is crucial for maintaining the integrity of the titration process. By employing best practices and modern advancements in titration technology, laboratories can ensure accurate and efficient moisture analysis.
As industries continue to evolve and prioritize product integrity, the relevance of Karl Fischer titration will only grow. Embracing this method and its intricacies allows professionals to uphold high standards of quality control, ultimately leading to safer and more effective products in the market. The significance of moisture analysis in ensuring compliance with regulatory standards cannot be overstated, cementing Karl Fischer titration’s role as a cornerstone in analytical chemistry.




