Overview
This article underscores the critical importance of pH buffer solutions in laboratory environments, highlighting their indispensable role in maintaining stable pH levels, which are essential for accurate measurements and dependable experimental results. pH buffers, which effectively resist changes in acidity or alkalinity, are vital across a range of applications. This is particularly true in pharmaceutical testing, where even minor fluctuations in pH can drastically influence the stability and efficacy of drug compounds. The necessity of employing high-quality pH buffers cannot be overstated, as they are fundamental to ensuring the integrity of scientific research and development.
Introduction
pH buffer solutions are essential for ensuring the accuracy and reliability of laboratory measurements, serving as critical safeguards against fluctuations that can undermine experimental integrity. These meticulously formulated mixtures not only stabilize pH levels during sensitive biochemical assays but also guarantee that vital reactions—particularly those involving enzymes—occur under optimal conditions. Yet, a significant challenge persists: how can laboratory professionals effectively select, prepare, and implement these buffers to adeptly navigate the complexities of varying pH levels? This article explores the fundamental components, various types, and best practices for mastering pH buffer solutions, ultimately enhancing the quality of scientific research.
Define pH Buffer Solutions and Their Importance in Laboratories
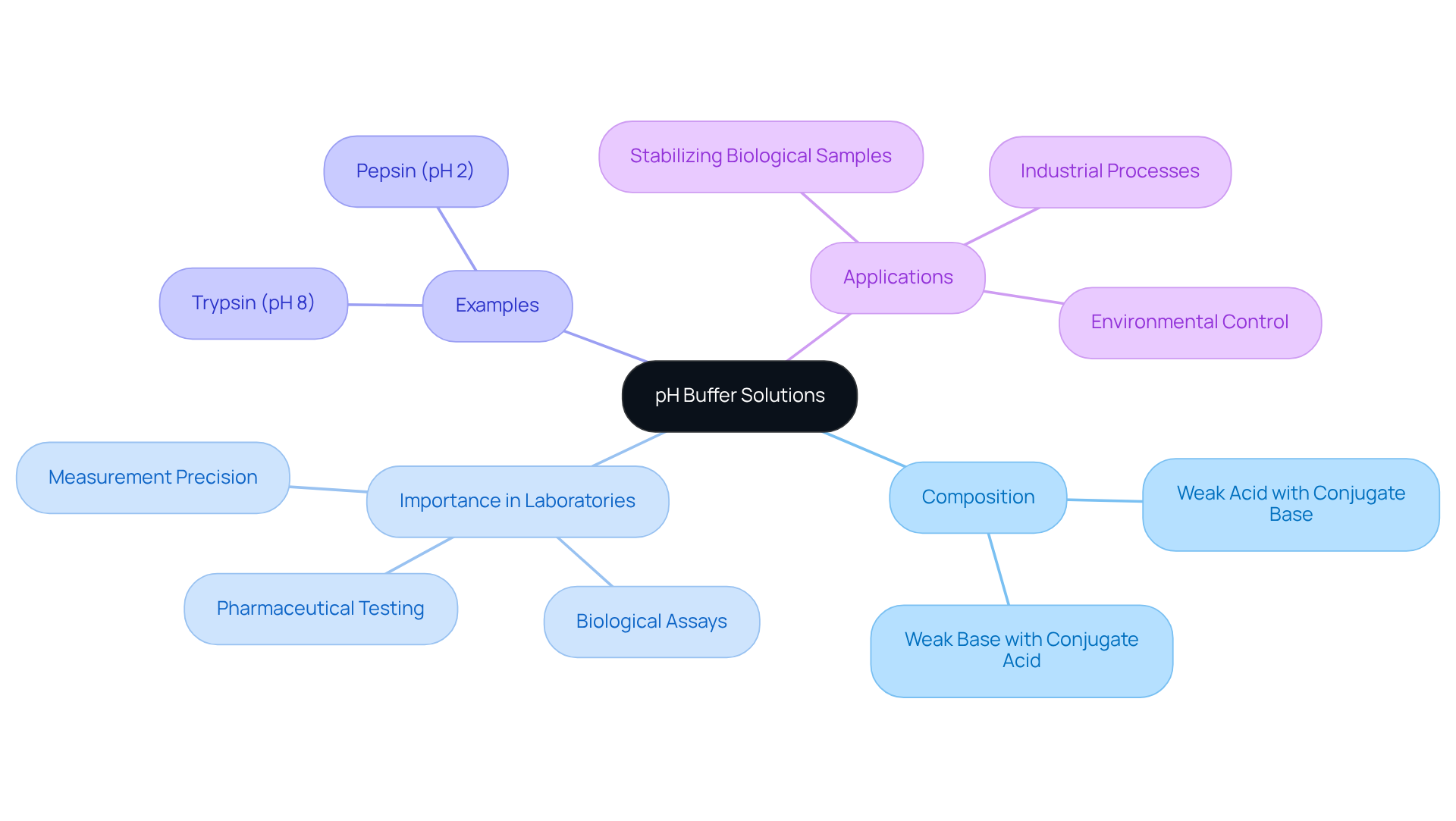
pH buffer solutions are essential water-based mixtures designed to resist changes in pH when small amounts of acids or bases are introduced. Typically composed of a weak acid paired with its conjugate base or a weak base with its conjugate acid, pH buffer solutions are indispensable in laboratory environments. Their significance is particularly pronounced in pharmaceutical testing, where the use of pH buffer solutions can dramatically influence the solubility and stability of drug compounds. For instance, trypsin, an enzyme vital for digestion, operates optimally at a pH of approximately 8, whereas pepsin thrives in acidic conditions near pH 2. This highlights the critical role that specific pH levels play in enzyme activity and, consequently, in the effectiveness of pharmaceutical formulations.
Moreover, pH buffer solutions are crucial for stabilizing mixtures to ensure that biological assays, which are highly sensitive to pH fluctuations, yield consistent and reliable results. A prime example is the bicarbonate buffering system in the human body, which maintains blood pH within a narrow range of 7.35 to 7.45—an essential factor for physiological processes such as oxygen transport and enzyme functionality. In laboratory settings, the use of pH buffer solutions is vital for maintaining the desired pH, which is crucial for the accuracy of analytical measurements, as even minor deviations can lead to significant variations in outcomes. Regular calibration of pH meters using reference fluids with known pH values is essential for ensuring measurement precision, as this practice helps mitigate errors that may arise due to instrument drift.
It is also important to recognize that neutralizing solutions can be overwhelmed by substantial amounts of strong substances or bases, leading to rapid pH changes that compromise their effectiveness. For effective stabilization, the ideal pKa of the weak acid should be within one pH unit of the target pH, ensuring that the pH buffer solutions can respond adequately to pH changes. In summary, the effective application of pH buffer solutions not only stabilizes chemical reactions but also enhances the overall quality of research work, making pH buffer solutions a cornerstone of reliable pharmaceutical testing and investigation.
Explore Types of pH Buffers and Their Components
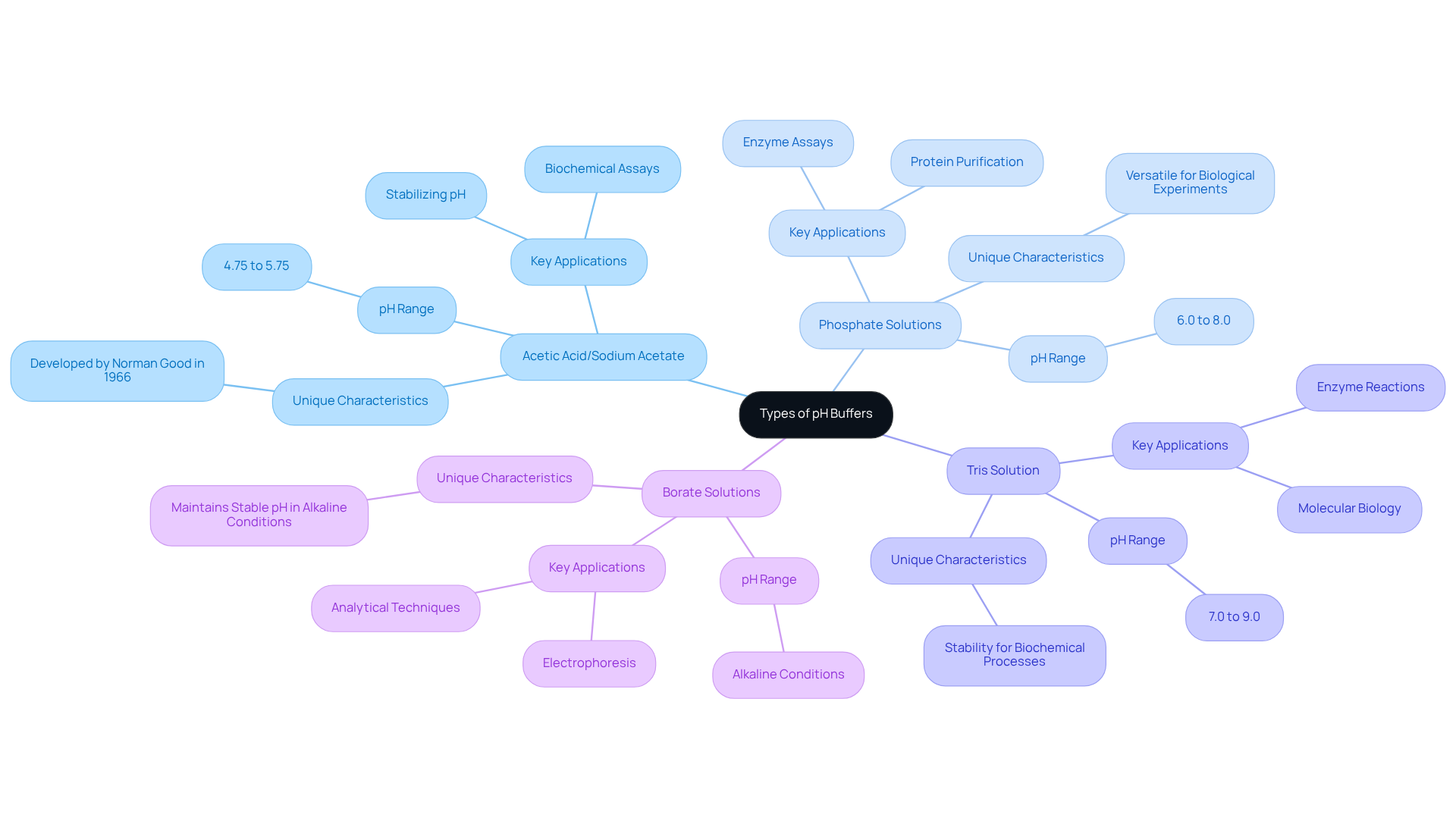
A range of pH buffer solutions is specifically designed for biochemical assays and laboratory environments, underscoring their critical role in scientific research. Key buffer systems include:
-
Acetic Acid/Sodium Acetate: This solution operates effectively within a pH range of 4.75 to 5.75 and is widely utilized in biochemical assays due to its ability to stabilize pH during reactions involving weak acids and bases. The foundational work of Norman Good in 1966 established parameters for efficient support systems, emphasizing their significance in biochemical research.
-
Phosphate Solutions: Comprising dihydrogen phosphate and hydrogen phosphate, these solutions regulate pH levels between 6.0 and 8.0. Their versatility renders them suitable for a variety of biological experiments, including enzyme assays and protein purification, where maintaining physiological pH is essential.
-
Tris Solution: Commonly employed in molecular biology, Tris (tris(hydroxymethyl)aminomethane) formulations provide stability in the pH range of 7.0 to 9.0. This medium is particularly effective for enzyme reactions, ensuring optimal conditions for biochemical processes.
-
Borate Solutions: Known for their usefulness in electrophoresis, borate solutions maintain stable pH levels in alkaline conditions, making them indispensable for various analytical techniques.
Each type of buffer is characterized by specific components that determine its capacity for absorption and pH range. Selecting the appropriate pH buffer solutions is crucial for achieving precise and consistent outcomes in laboratory experiments, especially in biochemical studies where pH stability is vital. The Henderson-Hasselbalch equation, which connects the pH of a solution to the concentrations of the weak acid and its conjugate base, serves as a fundamental concept in understanding the effective operation of these systems.
Prepare and Apply pH Buffers for Accurate Measurements
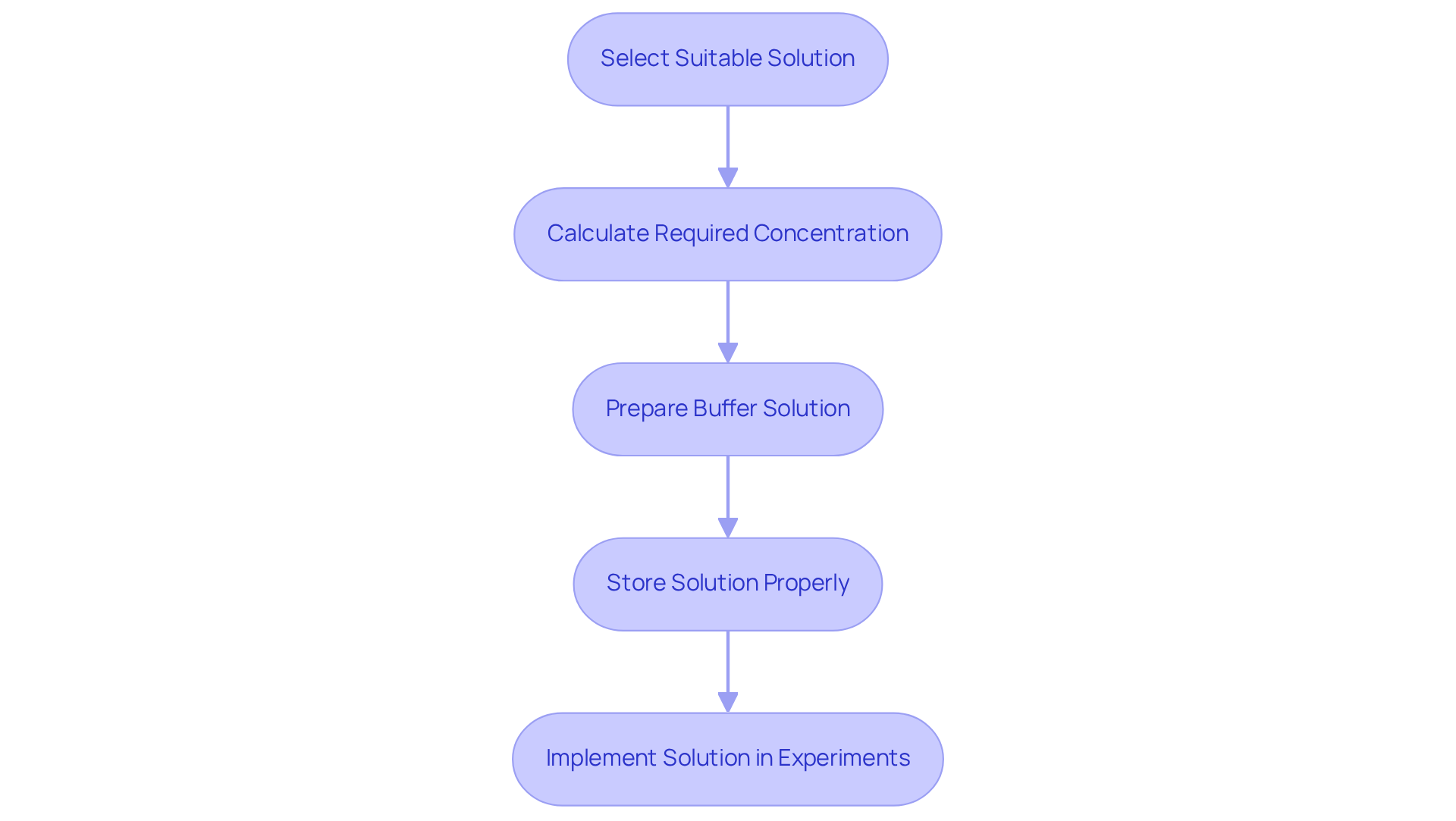
To prepare and apply pH buffers effectively, follow these essential steps:
-
Select the Suitable Solution: Choose a solution system based on the desired pH range and specific application. For instance, Good's pH buffer solutions are widely acknowledged for their effectiveness in preserving pH stability in biological experiments, particularly within the range of 6 to 8. As Andrew Porterfield observes, 'Choosing a stabilizing agent that will effectively maintain your experiment's pH is crucial to its success.'
-
Calculate the required concentration of pH buffer solutions: Determine the concentration needed for your application, typically ranging from 0.1 M to 1.0 M. This concentration is crucial for ensuring that pH buffer solutions can effectively resist pH changes during experiments. Higher concentrations generally provide better buffering capacity, which is vital for maintaining stable conditions in biological systems.
-
Prepare the Buffer Solution: Measure the appropriate amounts of the weak acid and its conjugate base (or vice versa) using an analytical balance for precision. Dissolve the components in distilled water and adjust the volume to the desired final volume. Utilize a pH meter to check the pH buffer solutions and make necessary adjustments by adding small amounts of acid or base. This step is vital, as biological systems are sensitive to pH changes, which can significantly impact experimental outcomes. As noted, biological systems are extremely sensitive to changes in pH buffer solutions, which can alter or even shut down your experiment.
-
Store the Solution Properly: Store prepared solutions in clean, labeled containers to prevent contamination. Glass or high-quality plastic containers are suggested to maintain the integrity of the solution.
-
Implement the Solution in Experiments: Ensure that the solution is at the correct temperature, as pH can vary with temperature. Use the buffer to calibrate instruments or as a medium for reactions, ensuring that the pH remains stable throughout the experiment. For example, when using TRIS buffered solutions, maintaining a consistent ionic strength is critical for accurate pH measurements, especially in varying salinity conditions.
By adhering to these best practices, laboratory professionals can ensure accurate and reliable pH buffer solutions, which are essential for the success of various biochemical experiments.
Understand Regulatory Standards and Best Practices for pH Buffer Use
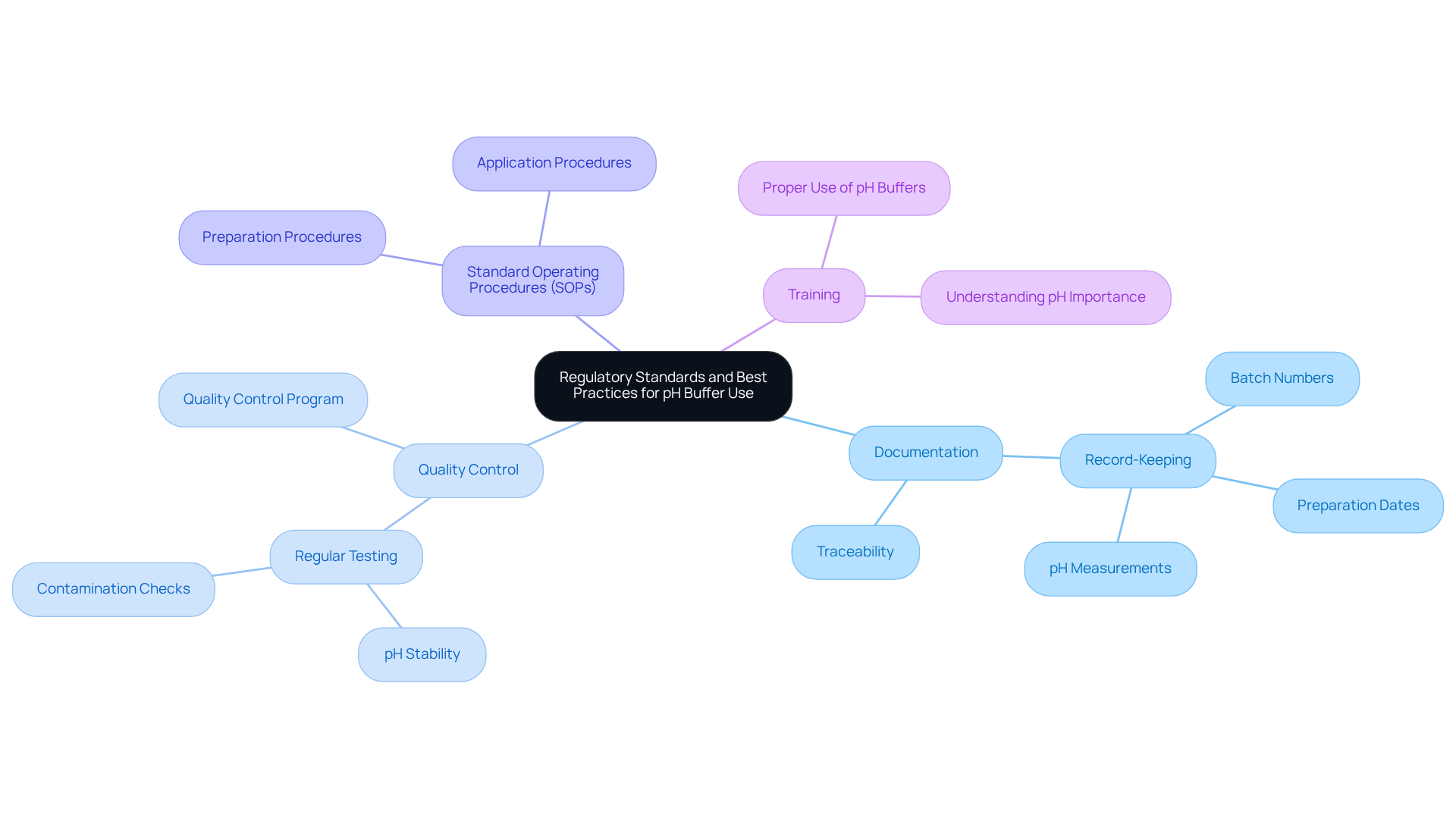
Laboratories are required to comply with specific regulatory standards when utilizing pH solutions, particularly in pharmaceutical and clinical environments. This compliance is crucial for ensuring the reliability of results and maintaining industry standards. Key considerations include documentation, quality control, standard operating procedures (SOPs), and training.
-
Documentation is essential. Laboratories must maintain precise records of solution preparation, including batch numbers, preparation dates, and pH measurements. This meticulous record-keeping ensures traceability and compliance with regulatory requirements.
-
Quality control cannot be overlooked. Regular testing of solution mixtures for pH stability and contamination is vital. Establishing a robust quality control program verifies that solutions consistently meet necessary specifications, safeguarding the integrity of laboratory results.
-
The creation and adherence to standard operating procedures (SOPs) for solution preparation and application are imperative. SOPs ensure consistency and reliability in experimental practices, minimizing the risk of errors that could compromise outcomes.
-
Training is a critical component. All staff in the laboratory must be adequately trained in the proper use of pH buffers and understand the significance of maintaining pH levels for accurate measurements. This knowledge empowers staff to uphold the highest standards in their work.
By following these best practices and regulatory standards, laboratories can significantly enhance the reliability of their results while ensuring compliance with industry regulations.
Conclusion
pH buffer solutions are essential for achieving accurate and reliable measurements in laboratory environments. By stabilizing pH levels, these solutions ensure the integrity of chemical reactions, particularly in sensitive contexts such as pharmaceutical testing. The importance of pH buffers transcends mere measurement; they are integral to enzyme activity and the overall success of biochemical experiments.
The article delineates various types of pH buffers, including:
- Acetic acid/sodium acetate
- Phosphate solutions
- Tris solutions
- Borate solutions
Each is specifically designed for distinct applications. It underscores the necessity of selecting the appropriate buffer based on the desired pH range and emphasizes the meticulous preparation and storage required to preserve their effectiveness. Moreover, adherence to regulatory standards and best practices is crucial for ensuring the reliability of laboratory results, highlighting the importance of proper documentation, quality control, and staff training.
In summary, mastering the use of pH buffer solutions is imperative for any laboratory striving for precision in their experiments. By comprehending their composition, applications, and the regulatory framework governing their use, laboratory professionals can significantly enhance the quality of their work. Embracing these practices not only fosters accuracy in measurements but also contributes to the overarching goals of scientific integrity and innovation in research.




