Overview
This article delineates best practices for lab managers concerning the utilization of pH calibration buffers, a critical element in ensuring precise pH measurements. It underscores the necessity of selecting suitable buffers, executing effective calibration procedures, and maintaining pH meters. These practices not only enhance the reliability of measurements but also extend the lifespan of the equipment, thereby fostering a more efficient laboratory environment. By adhering to these guidelines, lab managers can significantly improve their measurement accuracy and operational effectiveness.
Introduction
pH calibration buffers are essential for ensuring the accuracy and reliability of pH measurements in laboratory settings. By providing stable reference points, these buffers not only enhance the precision of readings but also safeguard the integrity of experimental results.
However, selecting and implementing these buffers can present significant challenges. What best practices can lab managers adopt to ensure optimal calibration?
This article explores essential strategies for mastering pH calibration buffers, from selecting the right solutions to effective calibration procedures, ultimately empowering lab managers to maintain peak performance in their pH measurement processes.
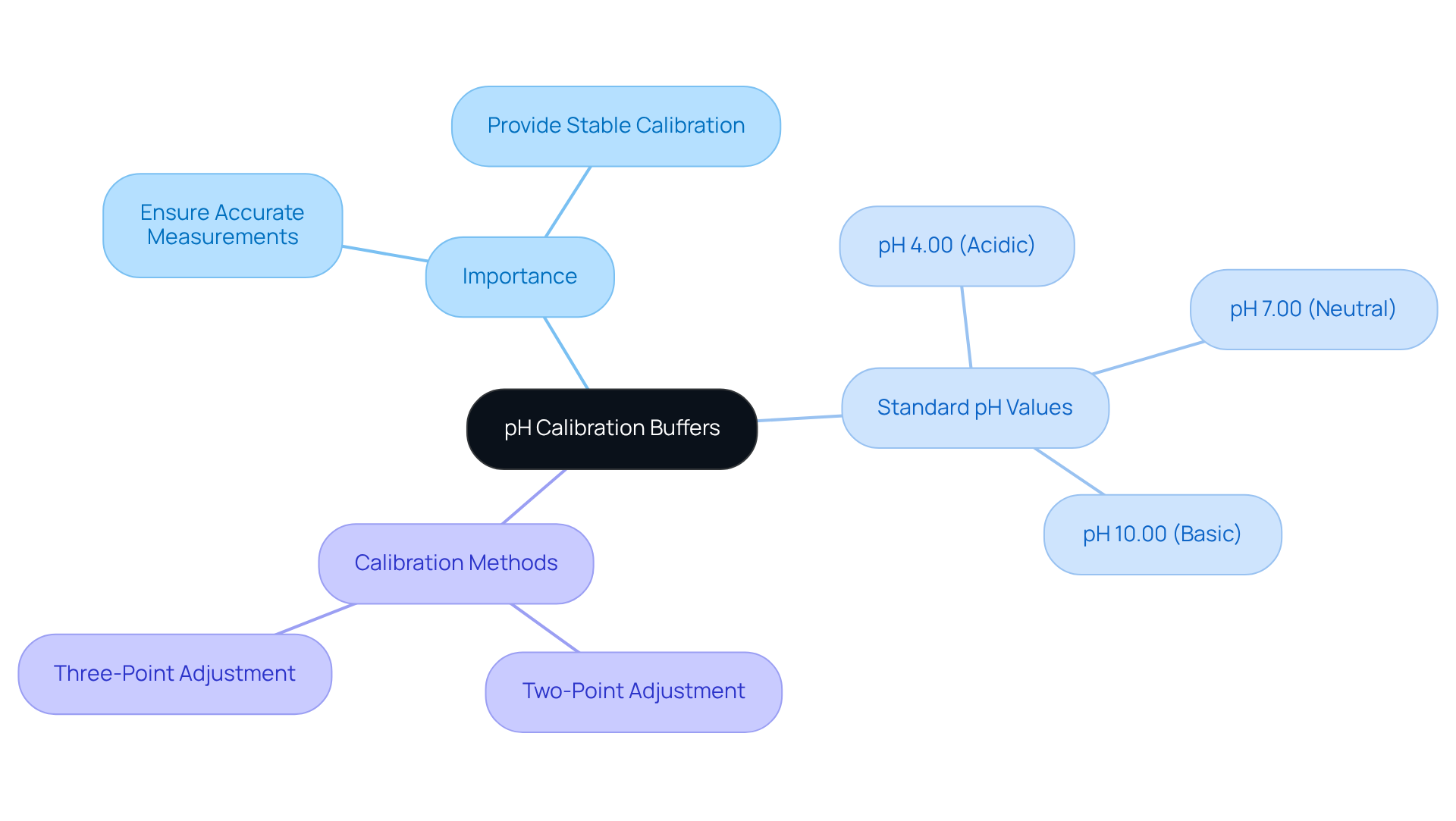
Understand pH Calibration Buffers: Importance and Functionality
pH calibration buffers are essential liquids with a known pH value, specifically designed to adjust pH instruments. Their primary function is to provide stable pH calibration buffers, which ensure accurate pH measurements. The importance of utilizing pH calibration buffers cannot be overstated; they are critical in ensuring that pH meter readings remain dependable and stable.
Typically, pH calibration buffers are offered in three standard pH values:
- 4.00
- 7.00
- 10.00
These values represent the acidic, neutral, and basic ranges, respectively. Employing these solutions with pH calibration buffers allows for either a two-point or three-point adjustment, which is vital for achieving high precision in pH measurements. For instance, a common practice is to perform a two-point adjustment using pH 4 and pH 7 solutions for acidic samples, while a three-point setup may include pH 10 for alkaline samples. Understanding the role of pH calibration buffers is the first step in establishing a robust adjustment procedure in any laboratory environment.
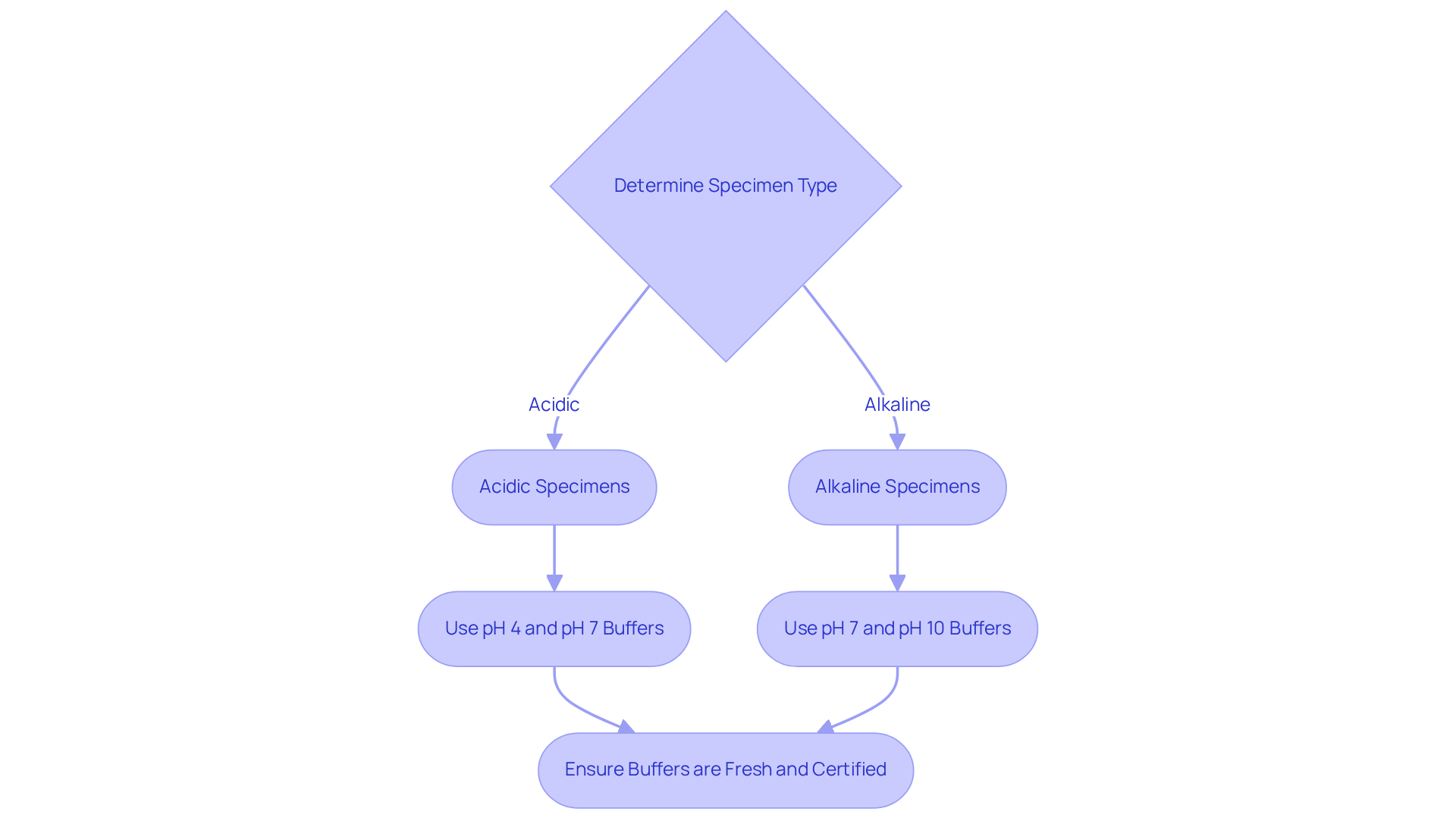
Select Appropriate pH Calibration Buffers for Accurate Measurements
Selecting appropriate pH adjustment solutions is crucial for ensuring accurate measurements in laboratory settings. Laboratory supervisors must consider the anticipated pH range of their specimens when making these selections.
For example, when dealing with acidic specimens, it is advisable to use pH calibration buffers of pH 4 and pH 7 for calibration. Conversely, for alkaline specimens, it is recommended to use pH calibration buffers of pH 7 and pH 10 solutions.
Furthermore, employing fresh, certified solutions is vital to prevent inaccuracies that may arise from expired or contaminated preparations. A best practice involves consistently maintaining a buffer that is slightly above and below the expected pH of the specimens being tested. This strategy not only enhances the precision of the adjustments but also ensures that the pH device remains responsive to the specific conditions of the samples under examination.
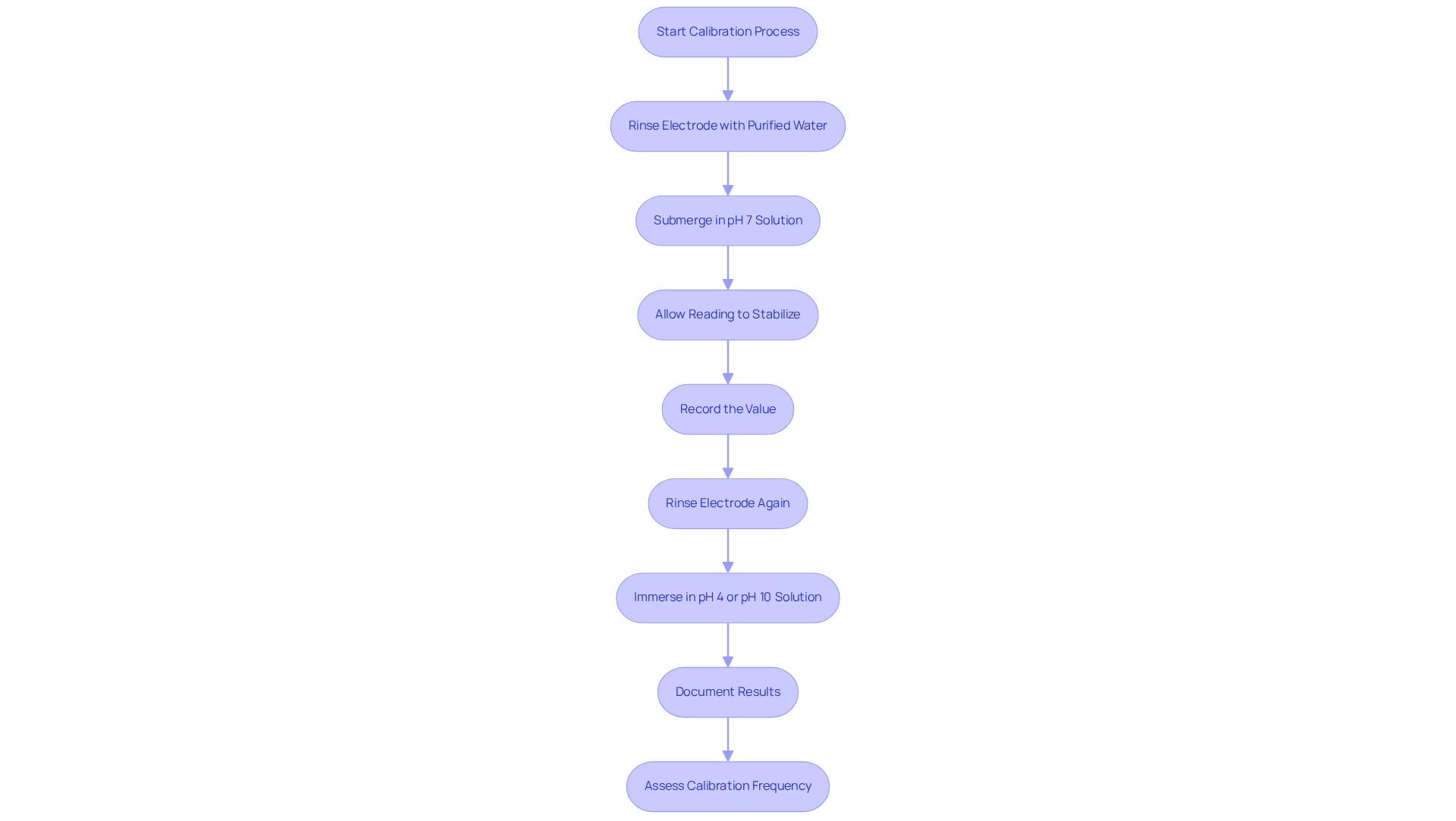
Implement Effective Calibration Procedures Using pH Buffers
Establishing effective adjustment procedures is crucial for ensuring the accuracy of pH measurements. To maintain high-quality results, it is recommended to perform a two-point adjustment using pH calibration buffers before each use of the pH meter.
Begin by rinsing the electrode with purified water, then submerge it in the initial standard solution, typically pH 7. Allow the reading to stabilize before recording the value. Next, rinse the electrode again and immerse it in the second solution of pH calibration buffers, choosing either pH 4 or pH 10 based on the expected sample pH.
After completing the adjustment, it is vital to document the results, including the date, time, and the lot numbers of the pH calibration buffers used. Regularly assess the calibration frequency based on the pH device's usage; for applications requiring high accuracy, daily calibration is advisable.
By adhering to these procedures, you can ensure that the pH device consistently delivers dependable and precise readings.
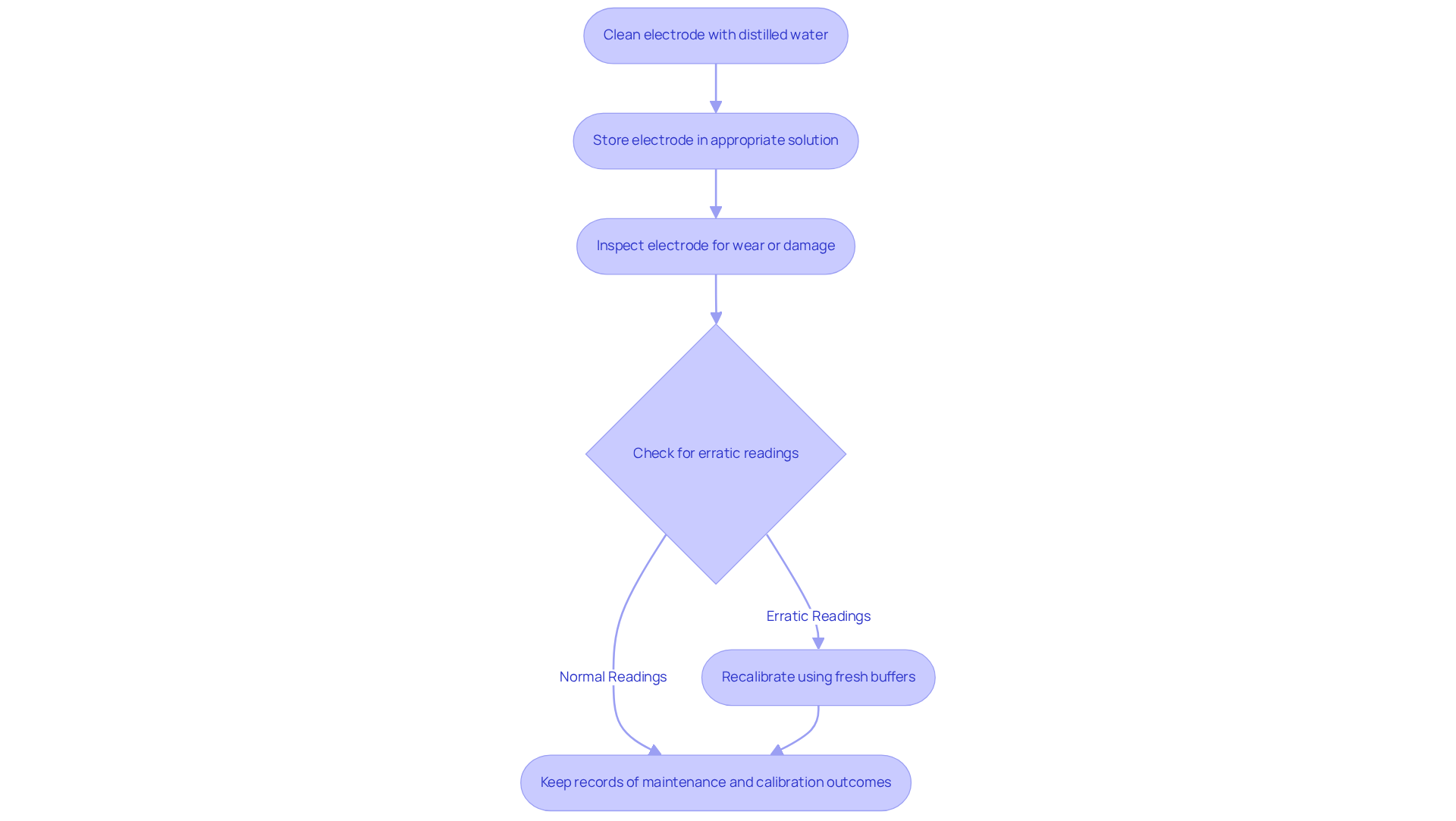
Maintain and Troubleshoot pH Meters for Consistent Performance
Maintaining and troubleshooting pH devices is essential for ensuring consistent performance in laboratory settings. Regular maintenance, such as cleaning the electrode with distilled water after each use and storing it in an appropriate solution to prevent drying out, is crucial. Lab managers must also periodically inspect the electrode for signs of wear or damage, including cracks or contamination. If erratic readings are observed from the pH instrument, it may indicate the need for cleaning or replacement of the electrode. A common troubleshooting step is recalibrating the device using fresh buffers to guarantee accuracy. Keeping a record of maintenance tasks and calibration outcomes is beneficial for recognizing trends that may indicate underlying issues with the pH device. By implementing these maintenance and troubleshooting practices, lab managers can significantly extend the lifespan of their pH meters while ensuring reliable performance in their laboratory operations.
Conclusion
Effectively utilizing pH calibration buffers is essential for achieving precise measurements in laboratory environments. These buffers not only stabilize pH readings but also underpin accurate calibration procedures that are crucial for reliable data collection. By comprehending their functionality and selecting the appropriate buffers, lab managers can significantly enhance the performance of their pH meters.
This article underscores the critical aspects of pH calibration, including:
- The careful selection of buffers based on the anticipated pH range of specimens
- The implementation of effective calibration procedures
- The importance of regular maintenance and troubleshooting of pH meters
Each of these elements is vital in ensuring that pH measurements are dependable and that laboratory operations proceed without disruption.
In summary, the importance of adhering to best practices for using pH calibration buffers cannot be overstated. By prioritizing proper selection, calibration, and maintenance, laboratory managers can ensure not only accurate measurements but also extend the lifespan of their equipment. Embracing these practices cultivates a culture of precision and reliability in laboratory work, ultimately leading to improved scientific outcomes.




