Overview
The article underscores the critical importance of proper pH meter storage solutions to guarantee accurate laboratory results, emphasizing the necessity of maintaining the hydration and functionality of pH electrodes. Best practices for storage are detailed, including:
- The use of specialized solutions rather than distilled water
- The regular monitoring of conditions
- Strict adherence to calibration schedules
These measures are essential to prevent common mistakes that can jeopardize measurement reliability. By implementing these strategies, laboratories can significantly enhance the precision of their results, reinforcing the importance of investing in high-quality scientific instruments.
Introduction
The accuracy of laboratory results is fundamentally dependent on the meticulous care of essential tools such as pH meters, which are indispensable for measuring the acidity or alkalinity of solutions. Recognizing the importance of effective pH meter storage solutions not only prolongs the lifespan of these delicate instruments but also guarantees reliable measurements that are critical for compliance across various scientific fields.
Despite this, many laboratories frequently overlook common storage pitfalls that jeopardize performance. What best practices can be implemented to safeguard these vital components and prevent costly errors?
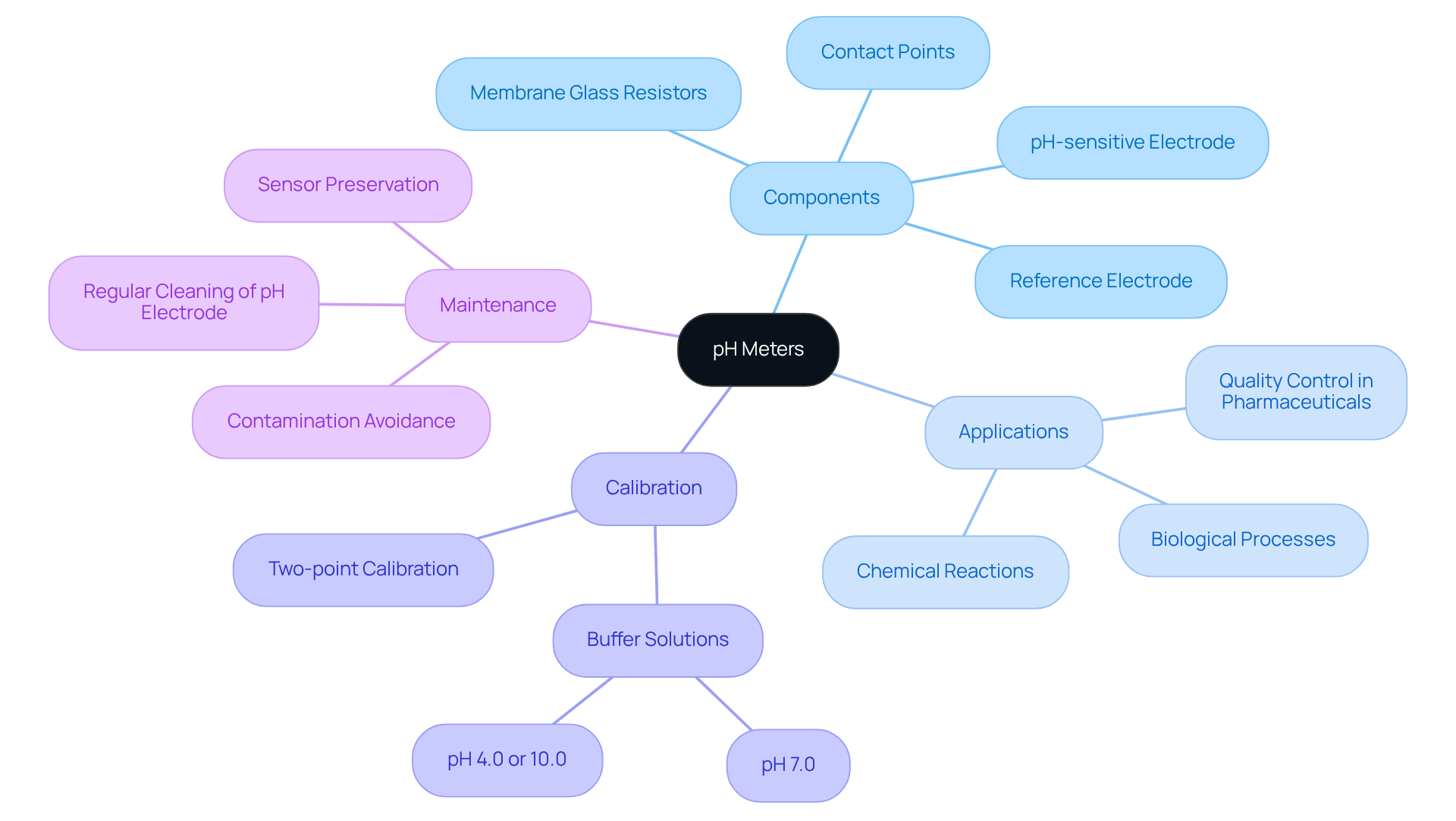
Explore the Basics of pH Meters and Their Significance in Laboratories
pH meters are essential instruments for measuring the acidity or alkalinity of a solution, expressed in pH units. Comprising a pH-sensitive electrode and a reference electrode, these devices work together to provide accurate readings. In laboratory settings, precise pH measurements are critical for various applications, including:
- Chemical reactions
- Biological processes
- Quality control in pharmaceuticals
Understanding the significance of pH meters enables laboratory personnel to ensure their readings are reliable, which is vital for maintaining compliance with regulatory standards and achieving successful outcomes in research and diagnostics. The proper use and maintenance of these instruments directly influence the quality of laboratory results, emphasizing the necessity for users to be well-informed about their operation and care.
Beyond the conventional glass membrane models, ISFET (Ion-Sensitive Field-Effect Transistor) pH meters are available, offering enhanced stability and performance for specific applications. Calibration is a fundamental aspect of pH meter operation; it is advisable to conduct a two-point calibration using buffer solutions with pH values of 7.0 and either 4.0 or 10.0 before initial use and at least annually thereafter. This practice ensures accuracy in readings, which can achieve precision levels of 0.01 pH.
Recent advancements in pH meter technology, including digital models that facilitate automatic calibration with the push of a button, have significantly enhanced usability and reliability. A solid grasp of the electrochemical principles underlying pH meters, such as the Nernst equation that connects ion concentration to pH value, is crucial for laboratory staff to prevent measurement errors. The electrical potential at the glass sensor is directly affected by the concentration of H+ ions, making the maintenance of the pH sensor essential to avoid contamination and ensure accurate readings. Furthermore, proper preservation of pH sensors in a pH meter storage solution is vital to maintain their functionality over time.
In pharmaceuticals, real-world applications of pH meters include monitoring the pH of drug formulations and ensuring adherence to regulatory standards. The components of pH meters, such as membrane glass resistors and contact points, are integral to their operation. By thoroughly understanding these elements and following best practices for usage and maintenance, laboratory personnel can significantly improve the reliability of their pH measurements, ultimately contributing to successful outcomes in research and diagnostics.
Understand the Importance of pH Electrode Storage Solutions
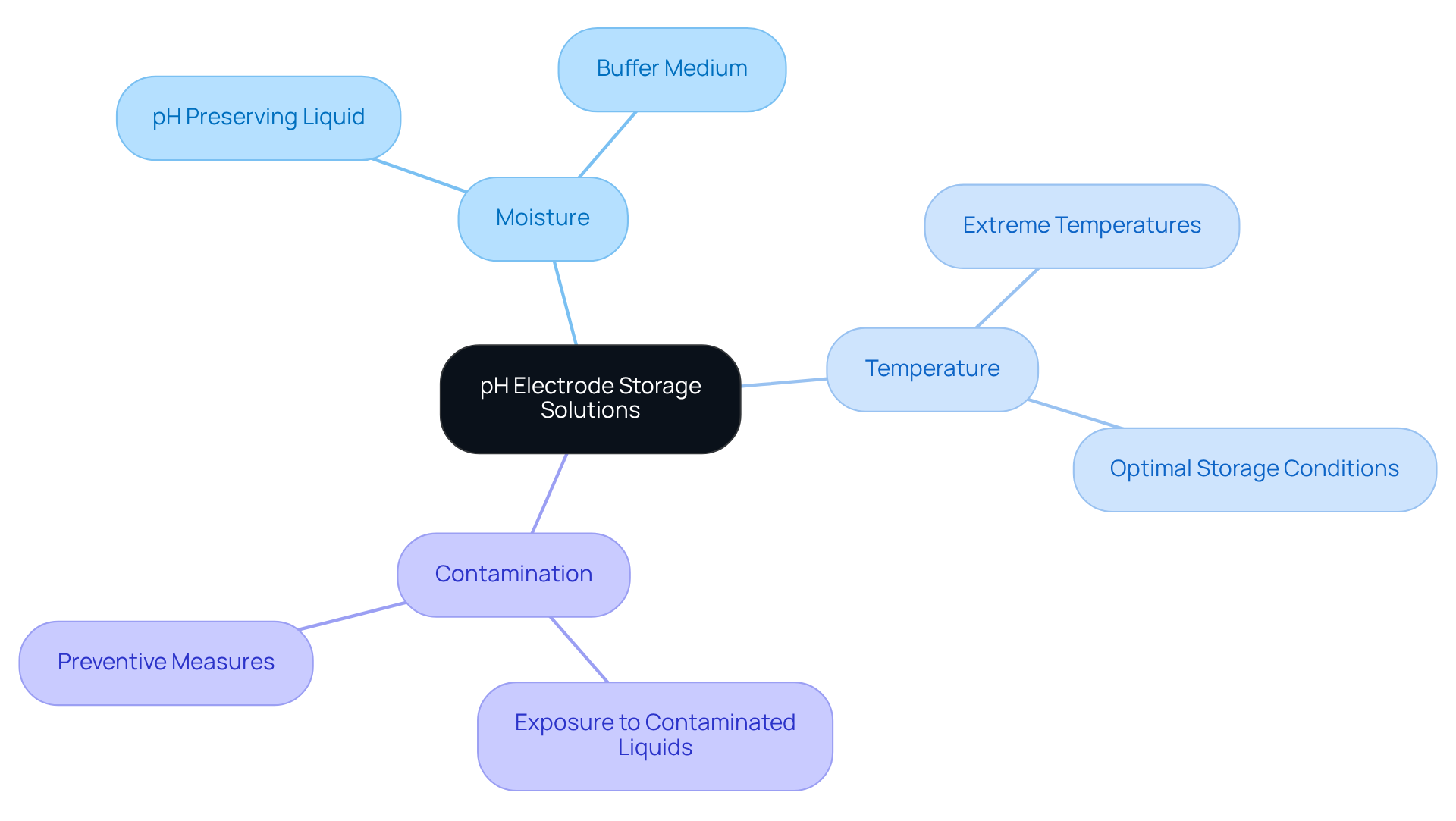
pH sensors are delicate components that require specific environmental conditions to maintain their functionality. Proper methods of keeping these components moist prevent desiccation, which can lead to erroneous measurements and potential damage. By storing sensors in a pH meter storage solution, such as a pH preserving liquid or a buffer medium, laboratories can ensure that the glass membrane remains hydrated and operational.
Additionally, understanding the significance of temperature and contamination in preservation conditions is crucial. For instance, exposing sensors to extreme temperatures or contaminated liquids can diminish their effectiveness over time. By emphasizing suitable preservation methods, laboratories not only extend the lifespan of their pH sensors but also guarantee reliable precision in their measurements.
Implement Best Practices for Storing pH Electrodes
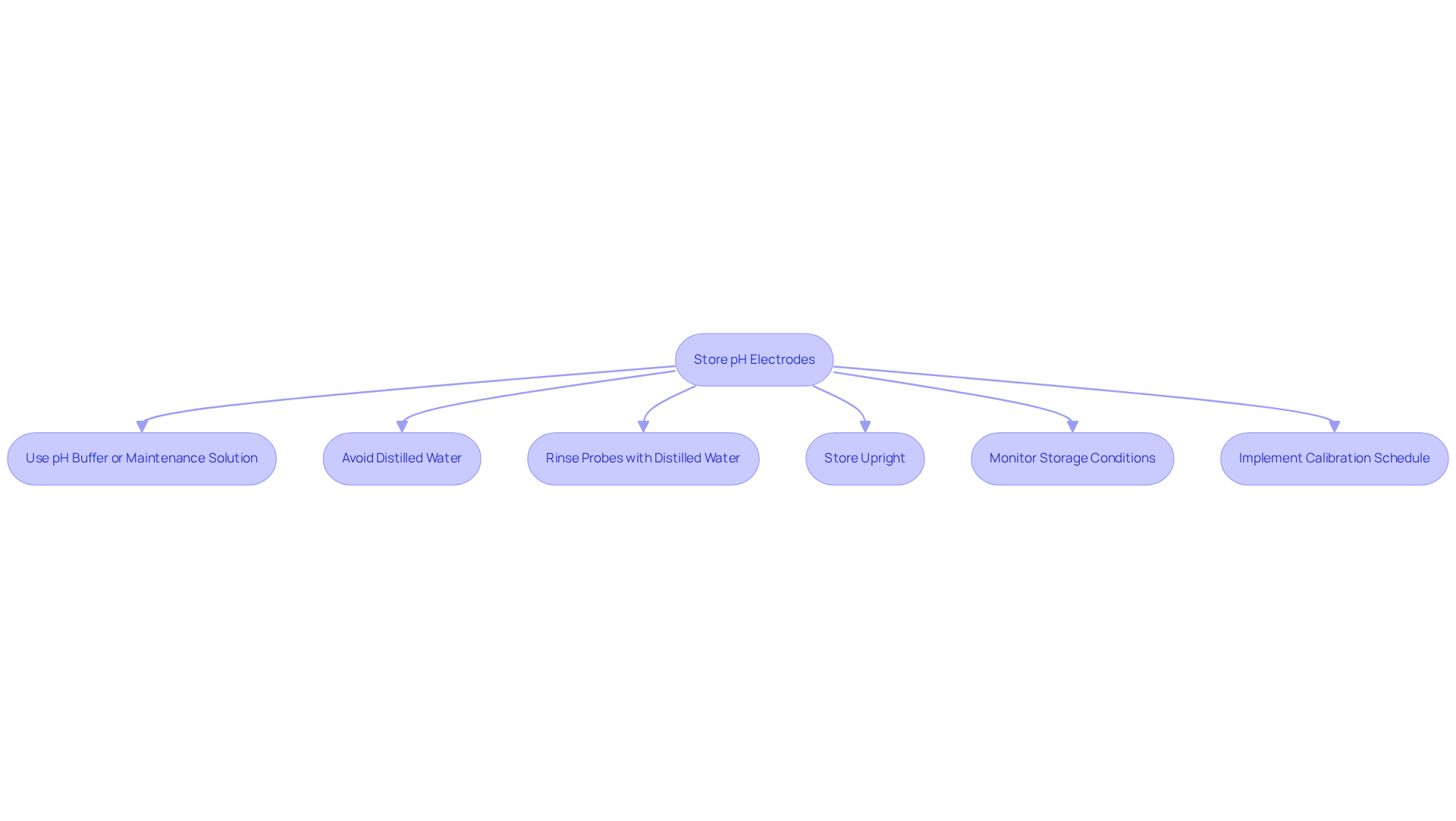
To ensure the longevity and accuracy of pH electrodes, following best practices for a pH meter storage solution is essential. Proper preservation is critical; always keep pH sensors in a pH meter storage solution, such as a pH buffer or a specialized sensor maintenance solution, to maintain the hydration of the glass membrane. Avoid storing sensors in distilled water, as this can lead to the leaching of ions from the glass membrane, resulting in inaccurate readings. Additionally, maintaining the cleanliness of probes is vital—rinse them with distilled water before placing them in the pH meter storage solution to eliminate any impurities that might impact functionality.
Storing the components upright is also advisable. If feasible, keep them in a vertical position within their solution to prevent the glass bulb from making contact with the container's bottom, which may cause damage. It is equally important to monitor the conditions of the pH meter storage solution; regularly check for temperature and contamination to ensure optimal conditions for the longevity of the components. Furthermore, implementing a routine calibration schedule is necessary to ensure that the electrodes function correctly, even when stored properly.
By adhering to these best practices, laboratory personnel can significantly enhance the reliability and accuracy of their pH measurements.
Identify and Avoid Common Mistakes in pH Meter Storage
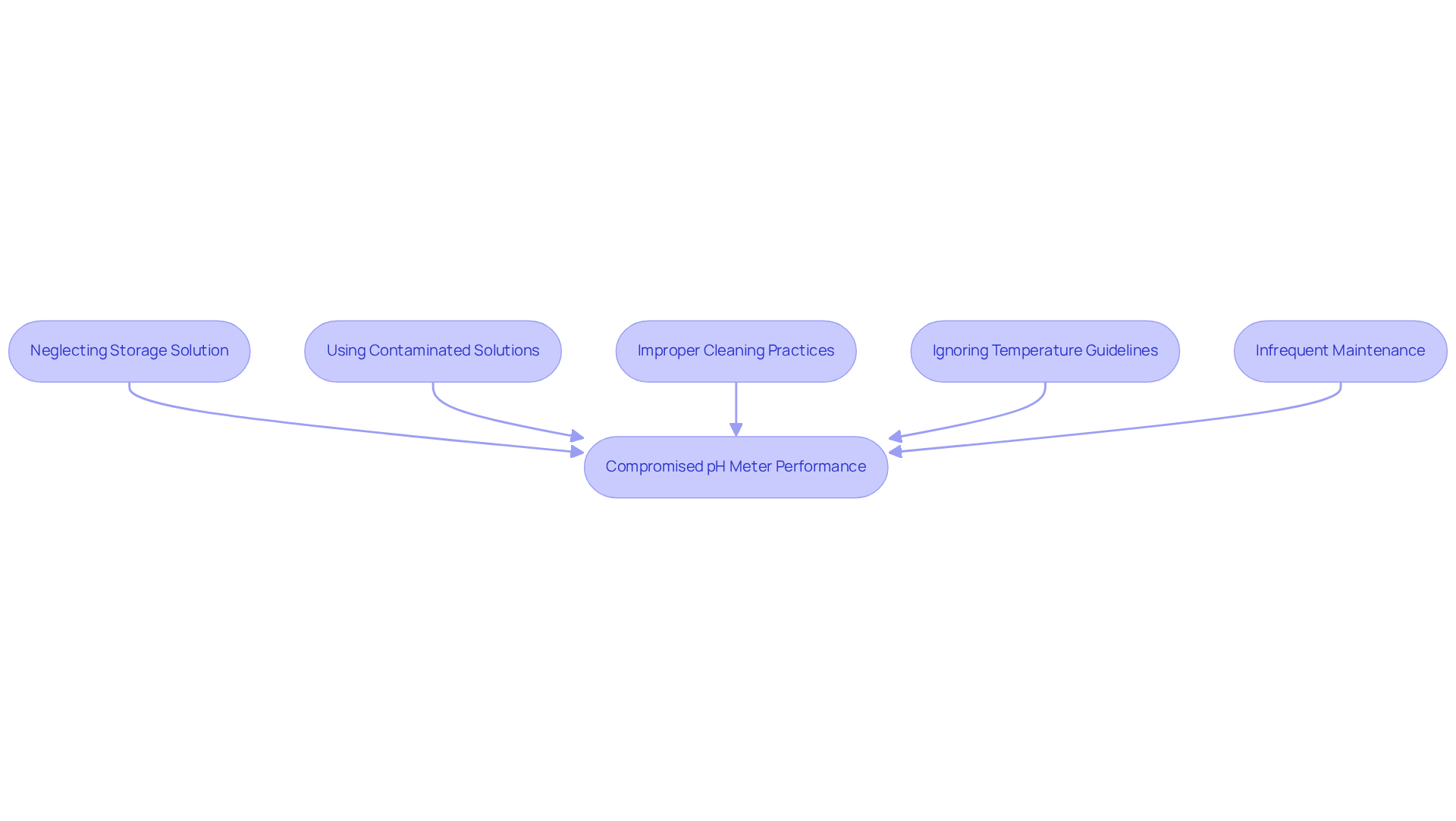
To ensure the accuracy and longevity of pH meters, it is crucial to utilize a pH meter storage solution to avoid common storage mistakes that can compromise their performance.
Neglecting to use a pH meter storage solution for preserving sensors in liquid is a primary concern; failing to do so can lead to desiccation and irreversible damage. Furthermore, using contaminated solutions can severely impact sensor performance, resulting in inaccurate readings that undermine laboratory results. Improper cleaning practices, such as neglecting to rinse components before storage, can leave residues that affect future measurements. Additionally, ignoring temperature guidelines can degrade the materials of these components, ultimately affecting their functionality. Lastly, infrequent maintenance can allow unnoticed degradation of electrode performance, leading to significant issues over time.
By recognizing these common pitfalls and implementing corrective measures, laboratory personnel can ensure the reliability of their pH meter storage solution, making them essential tools for precise measurements.
Conclusion
The integrity and performance of pH meters are paramount for achieving accurate laboratory results. This article underscores the critical importance of proper pH meter storage solutions, essential for preserving the functionality of pH electrodes. By understanding and implementing effective storage techniques, laboratory personnel can ensure that these vital instruments deliver reliable measurements, thus supporting successful research and diagnostics.
Key insights discussed include the necessity of keeping pH sensors in appropriate storage solutions to prevent desiccation and contamination. Best practices, such as:
- Avoiding distilled water
- Rinsing electrodes prior to storage
- Monitoring environmental conditions
are emphasized as critical steps in maintaining the longevity and accuracy of pH meters. Awareness of common mistakes, such as neglecting routine maintenance and using contaminated solutions, further highlights the importance of diligent care in laboratory settings.
Ultimately, the significance of proper pH meter storage extends beyond mere instrument maintenance; it directly impacts the quality of laboratory results and compliance with regulatory standards. By prioritizing these best practices, laboratory personnel can enhance the reliability of their measurements and contribute to the advancement of scientific research. Implementing these strategies not only safeguards the functionality of pH meters but also reinforces the overall integrity of laboratory operations.




