Overview
Potentiometric titration stands as a pivotal analytical method, essential for determining the concentration of unknown liquids through voltage measurements during titration. This technique not only provides precise endpoint detection without the need for visual indicators but also plays a critical role in the pharmaceutical industry, where accuracy and reliability are paramount.
The advantages of potentiometric titration are manifold:
- It boasts high sensitivity
- Versatility across various chemical reactions
- The potential for automation
All of which significantly enhance laboratory testing processes. As such, the importance of this method cannot be overstated, as it continues to shape the landscape of analytical chemistry.
Introduction
In the realm of analytical chemistry, potentiometric titration emerges as a sophisticated technique that surpasses traditional methods. By measuring voltage changes within an electrochemical cell, this approach facilitates the precise determination of an unknown solution's concentration, independent of visual indicators. This characteristic makes it particularly advantageous for analyzing colored or turbid samples. Its importance is especially evident in the pharmaceutical industry, where accuracy is paramount for drug testing and adherence to regulatory standards.
As advancements in titration technology continue to unfold, it becomes essential for professionals to understand the various types, procedures, and benefits of potentiometric titration to enhance their analytical capabilities.
Define Potentiometric Titration and Its Importance
Potentiometric analysis is a crucial analytical method for determining the concentration of an unknown liquid by measuring the voltage (potential) of an electrochemical cell during the introduction of a titrant. This technique is particularly significant due to its ability to provide precise endpoint determination without relying on visual indicators, making it especially suitable for colored or turbid solutions. Its application is widespread in the pharmaceutical industry, where accuracy and reliability are of utmost importance.
In the realm of pharmaceutical testing, advanced measurement techniques, such as those provided by the Hiranuma Aquacounter AQV-300 Volumetric and AQ-300 Coulometric Karl Fischer Analyzers, significantly enhance the precision of moisture content analysis in drugs and medicines. These titrators not only ensure compliance with the stringent standards set by the Japanese Pharmacopoeia but also offer the capability to gauge potential variations, thereby enabling a more delicate and precise evaluation of the analyte's level compared to conventional methods. This reinforces the necessity for high-quality scientific instruments in laboratory settings, making potentiometric analysis an essential tool in the pharmaceutical sector. By employing such advanced techniques, laboratories can uphold the integrity of their testing processes and ensure the safety and efficacy of pharmaceutical products.

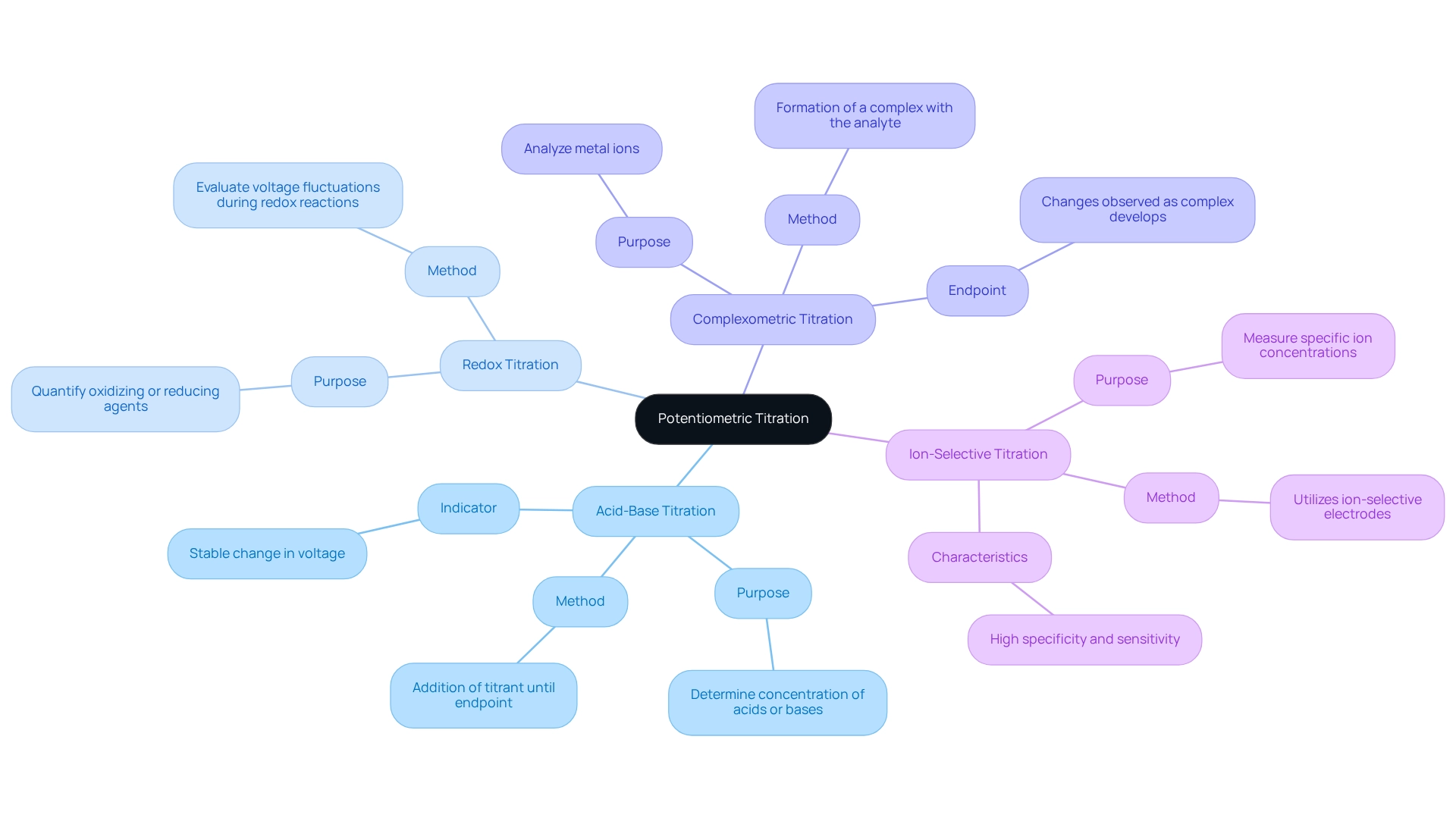
Explore Types of Potentiometric Titration
Potentiometric titration includes several distinct types, each fulfilling unique analytical purposes that are crucial in laboratory settings.
-
Acid-Base Titration stands as the most prevalent method, employed to ascertain the concentration of acids or bases within a solution. This process involves the careful addition of a titrant, typically a strong acid or base, to the analyte until the endpoint is reached. This endpoint is indicated by a stable change in voltage, providing a clear measure of concentration.
-
Redox Titration evaluates the fluctuations in voltage that occur during a redox reaction, characterized by the exchange of electrons between the analyte and titrant. This method is particularly useful for quantifying the presence of oxidizing or reducing agents, making it a valuable tool in various chemical analyses.
-
Complexometric Titration focuses on the formation of a complex between the analyte and the titrant, frequently applied in metal ion analysis. The endpoint of this titration is determined by observing the changes that occur as the complex develops, allowing for precise quantification of metal ions.
-
Ion-Selective Titration represents a specialized approach that utilizes ion-selective electrodes to measure the concentration of specific ions in a liquid. This method is renowned for its high specificity and sensitivity, making it indispensable in analytical chemistry.
In summary, understanding these types of potentiometric titrations is essential for effective analytical practices, allowing for precise measurements and improving the reliability of laboratory results.
Conduct Potentiometric Titration: Equipment and Procedure
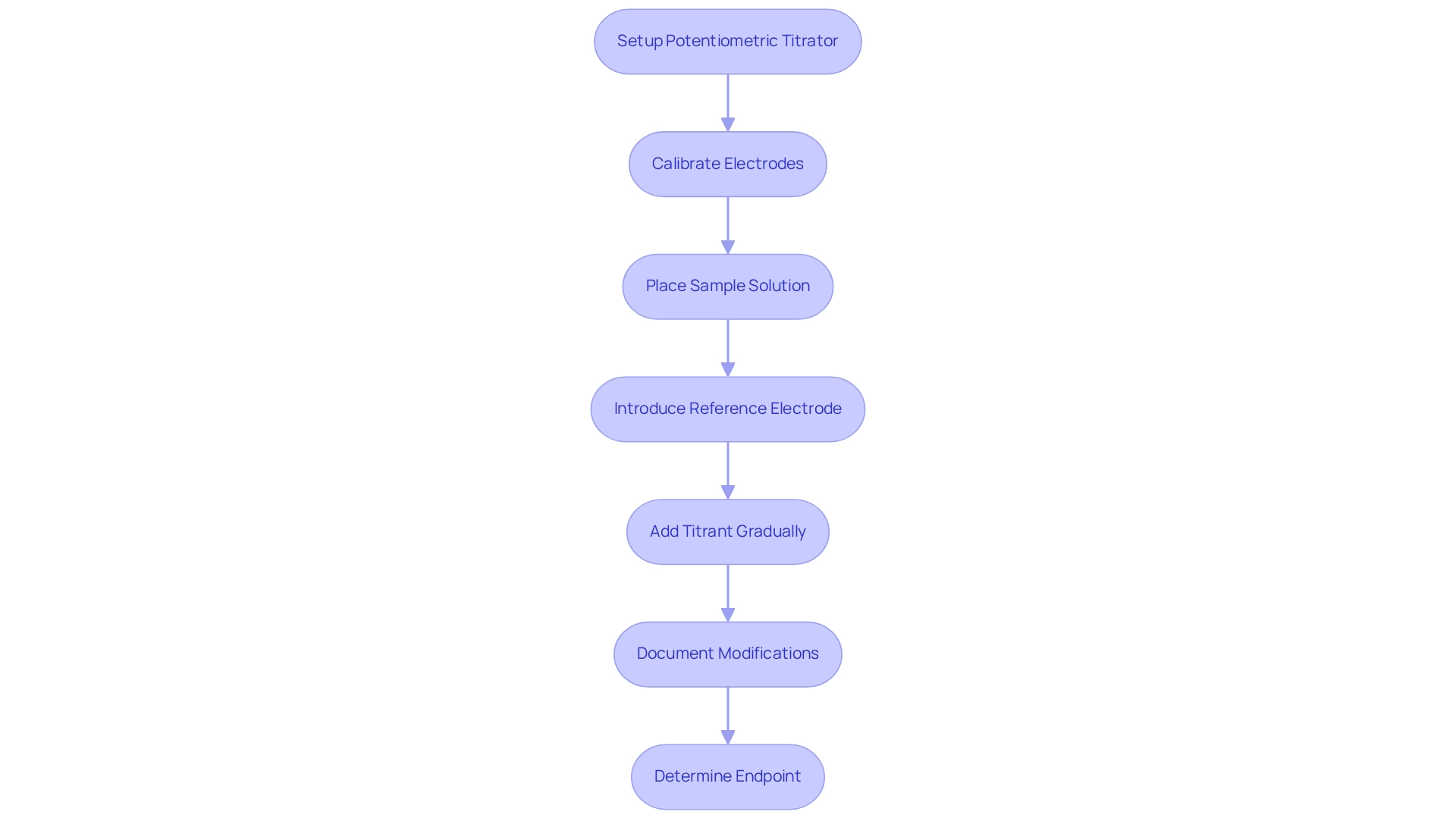
To conduct a potentiometric titration, it is essential to have specific equipment and materials to achieve accurate results. The potentiometric titrator, whether automated or manual, is crucial for accurately measuring the potential.
- Electrodes: A combination of a reference electrode, such as Ag/AgCl, and an indicator electrode, like a glass electrode for pH measurements, is necessary for effective analysis.
- Titrant: A liquid with a known strength is required to react with the analyte, ensuring precise measurements.
- Sample Result: The analyte liquid, whose concentration is to be determined, must be prepared adequately.
- Stirring Device: This tool is vital for ensuring thorough mixing during the titration process, which is essential for accuracy.
Procedure:
- Begin by setting up the potentiometric titrator and ensuring the electrodes are connected.
- Calibrate the electrodes according to the manufacturer's instructions to ensure reliable potentiometric readings.
- Place the sample solution in the measuring vessel and introduce the reference electrode.
- Initiate the titration by gradually adding the titrant while consistently monitoring the voltage.
- Document any modifications and chart them against the amount of titrant added to create a comprehensive curve.
- Finally, determine the endpoint by identifying the inflection point on the curve, where the potential changes rapidly, signaling the completion of the titration.
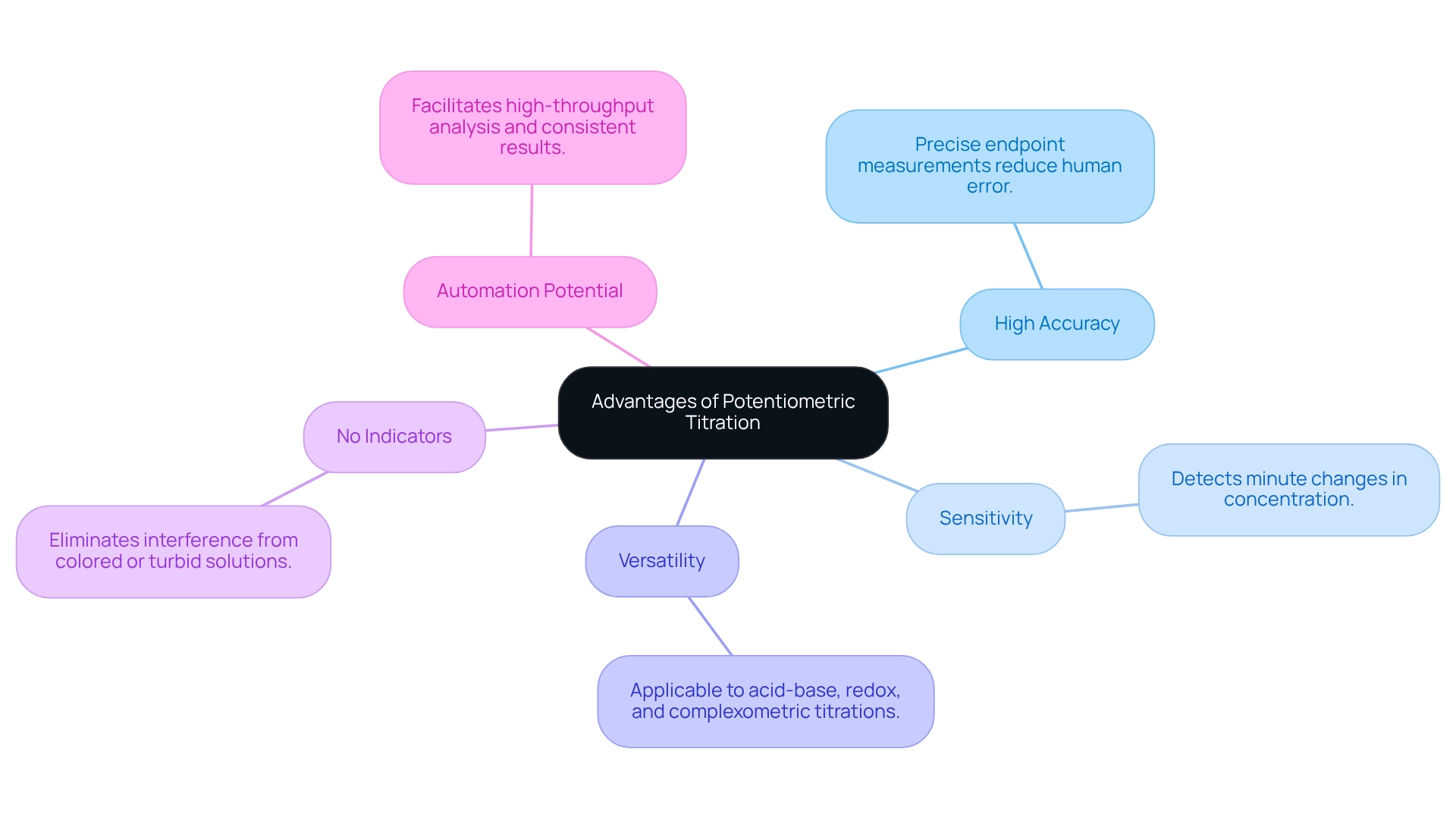
Evaluate Advantages of Potentiometric Titration
Potentiometric titration offers several notable advantages compared to traditional titration methods.
- High Accuracy is one of its primary benefits; this method delivers precise measurements of the endpoint, significantly reducing the risk of human error that often accompanies visual indicators.
- Moreover, its Sensitivity allows for the detection of minute changes in concentration, making it particularly suitable for trace examination.
- The versatility of potentiometric titration is remarkable, as it can be utilized across a wide array of chemical reactions, including acid-base, redox, and complexometric titrations.
- Another key advantage is that potentiometric methods require no indicators. This feature eliminates the potential interference from colored or turbid solutions, which can complicate visual endpoint determination.
- Additionally, the automation potential of many potentiometric devices cannot be overlooked. These automated systems facilitate high-throughput analysis and ensure consistent results, a significant advantage in pharmaceutical and environmental laboratories.
In summary, the advantages of potentiometric titration underscore its importance as a high-quality scientific instrument in laboratory settings.
Conclusion
The significance of potentiometric titration in analytical chemistry is profound. This technique excels in accurately determining the concentration of unknown solutions without relying on visual indicators, making it especially valuable for colored or turbid samples. Its applications within the pharmaceutical industry underscore the necessity for precision and adherence to regulatory standards, ensuring that drug testing meets the highest accuracy requirements.
Various types of potentiometric titration—such as acid-base, redox, complexometric, and ion-selective titrations—cater to distinct analytical needs, illustrating the method's versatility. The detailed procedures and specialized equipment involved facilitate meticulous execution and yield reliable results.
Moreover, the advantages of potentiometric titration over traditional methods, including superior accuracy, sensitivity, and the removal of visual indicators, reinforce its importance in contemporary laboratories. As technology advances, comprehending and employing these techniques will empower professionals to enhance their analytical capabilities, leading to improved outcomes across numerous fields, particularly in pharmaceuticals. Embracing potentiometric titration is a crucial step toward achieving greater accuracy and efficiency in analytical chemistry.




