Overview
This article delves into the essential steps and troubleshooting methods crucial for mastering redox titration in a laboratory setting. Understanding redox principles is paramount; it forms the foundation upon which accurate measurements are built. Gathering the necessary materials and following a precise titration procedure are vital actions that cannot be overlooked. Moreover, addressing common issues ensures that the integrity of results is maintained. These practices are not merely procedural; they are critical in achieving reliable analytical results that underscore the importance of precision in scientific inquiry.
Introduction
Mastering the art of redox titration transcends mere academic exercise; it is a fundamental skill integral to the accuracy of chemical analysis across various fields, especially in pharmaceuticals. This intricate process, anchored in the principles of oxidation and reduction reactions, serves as a gateway to precise measurements and reliable results. Yet, the journey to proficiency is not without its challenges, including:
- The misidentification of endpoints
- The risk of contamination
How can one adeptly navigate these complexities to ensure successful outcomes in the redox titration lab? Understanding these nuances is essential for anyone striving to achieve excellence in this critical area of study.
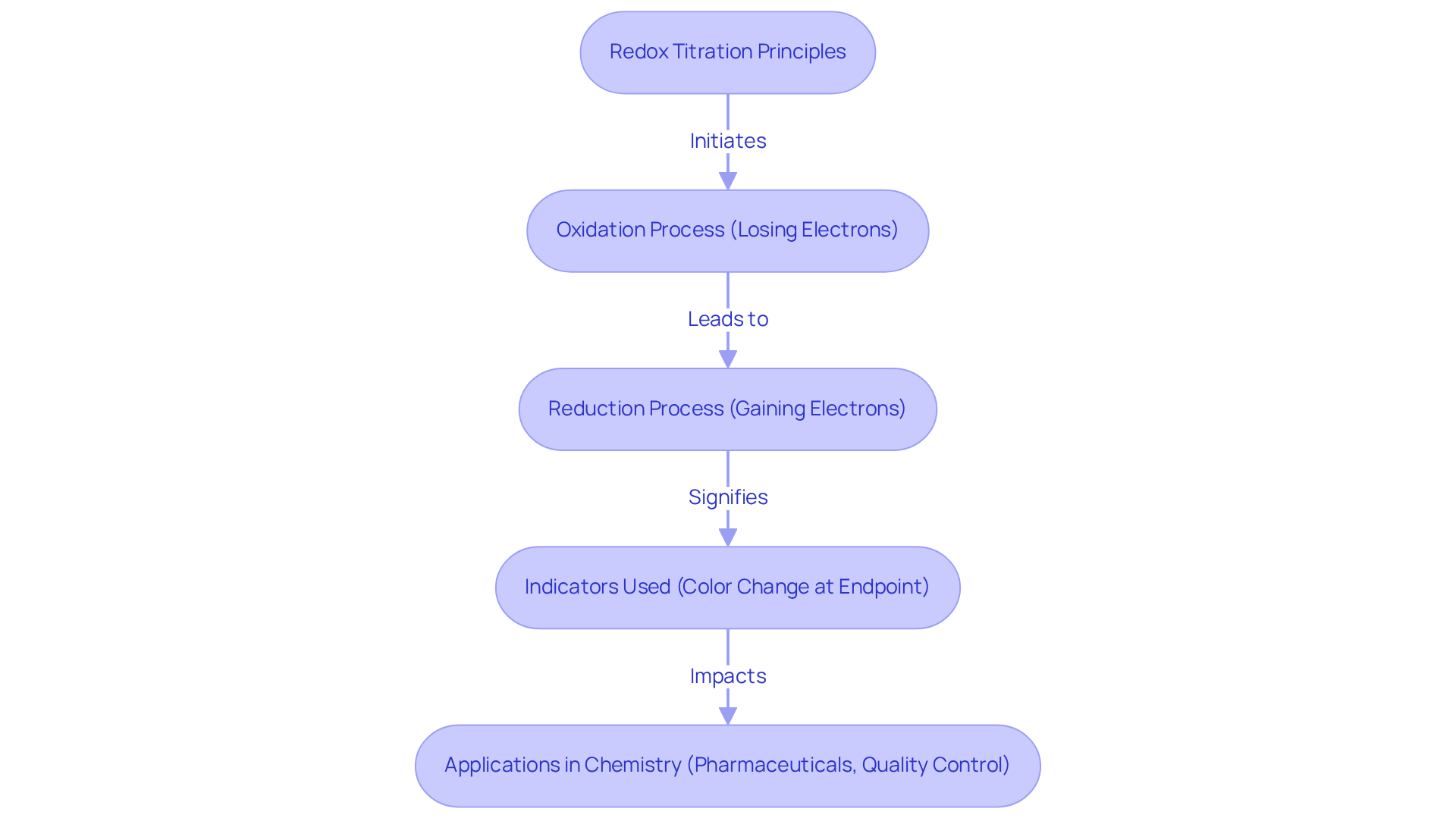
Understand Redox Titration Principles
The redox titration lab serves as , essential for determining the concentration of an unknown solution through its reaction with a reagent of known concentration via oxidation-reduction reactions. In this intricate process, one species undergoes oxidation—losing electrons—while another experiences reduction—gaining electrons. A comprehensive grasp of the half-reactions involved in a redox titration lab is crucial; it enables the calculation of the analyte's concentration based on the volume of titrant utilized. Leading chemists assert that mastering these half-reactions is foundational to excelling in the redox titration lab, as it underpins accurate analytical evaluations.
Common indicators, such as potassium permanganate, are noteworthy for their distinct color change at the endpoint, signifying the completion of the reaction. This understanding not only enhances your ability to conduct precise measurements but also aids in effectively interpreting results. Such proficiency is particularly vital in pharmaceutical laboratories and redox titration lab settings, where accurate calculations are paramount. The significance of comprehending redox reactions transcends laboratory confines, impacting various applications in analytical chemistry, including pharmaceutical development and quality control processes.
In summary, the mastery of redox analysis and its underlying principles is indispensable for professionals in the field. It not only facilitates accurate measurements but also supports the broader objectives of quality assurance and innovation in pharmaceutical practices.
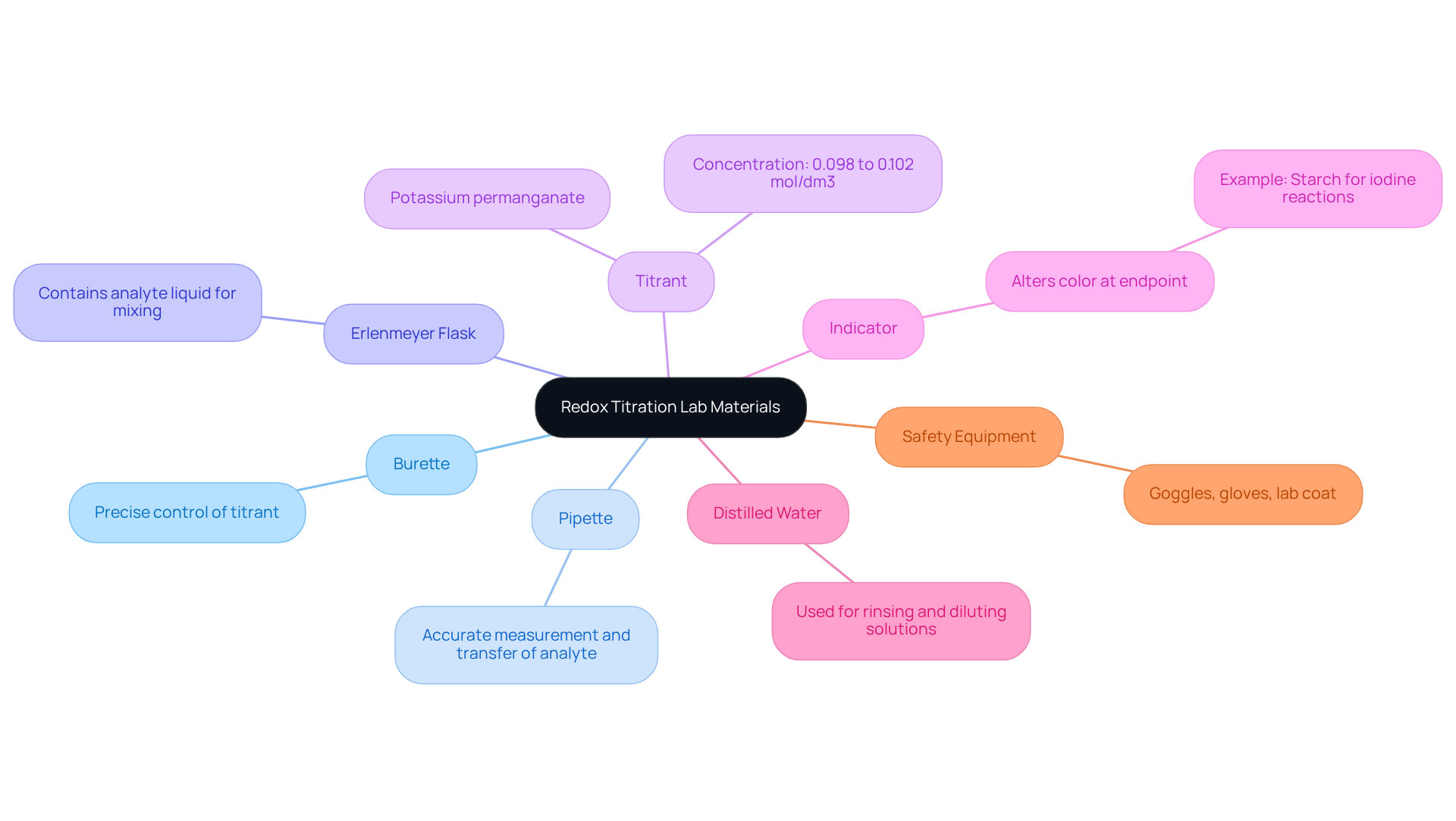
Gather Required Materials and Equipment
To effectively conduct a redox titration lab, it is essential to gather the following materials and equipment. First, the Burette is crucial for accurately dispensing the titrant, allowing for precise control over the titration process. Next, a Pipette is necessary to measure and transfer the analyte liquid with accuracy, ensuring reliable results. The Erlenmeyer Flask is perfect for containing the analyte liquid during the process, enabling easy mixing and observation of color changes. Additionally, a Titrant, a liquid of known concentration such as potassium permanganate, is essential for the titrating process. The certified value of potassium dichromate, for instance, has a molar concentration range of 0.098 mol/dm3 to 0.102 mol/dm3, with a relative expanded uncertainty of 0.024%, highlighting the importance of using standardized materials. Furthermore, selecting a suitable Indicator that alters color at the endpoint, like starch for iodine reactions, is critical to indicate the conclusion of the reaction. It is also vital to utilize Distilled Water for rinsing and diluting solutions to prevent contamination. Finally, always wear Safety Equipment such as goggles, gloves, and a lab coat to ensure safety during the experiment.
Prior to use, ensure that all equipment is thoroughly cleaned and calibrated. This step is vital to avoid contamination and inaccuracies, which can significantly affect the results of your titration. As indicated by specialists, ' ensures the accuracy of analytical measurements, serving as a cornerstone for the integrity of experimental results.' Adhering to these best practices not only enhances the reliability of your measurements but also aligns with current trends in laboratory practices, particularly in the context of a redox titration lab, which emphasizes the importance of precision and safety in scientific analysis.
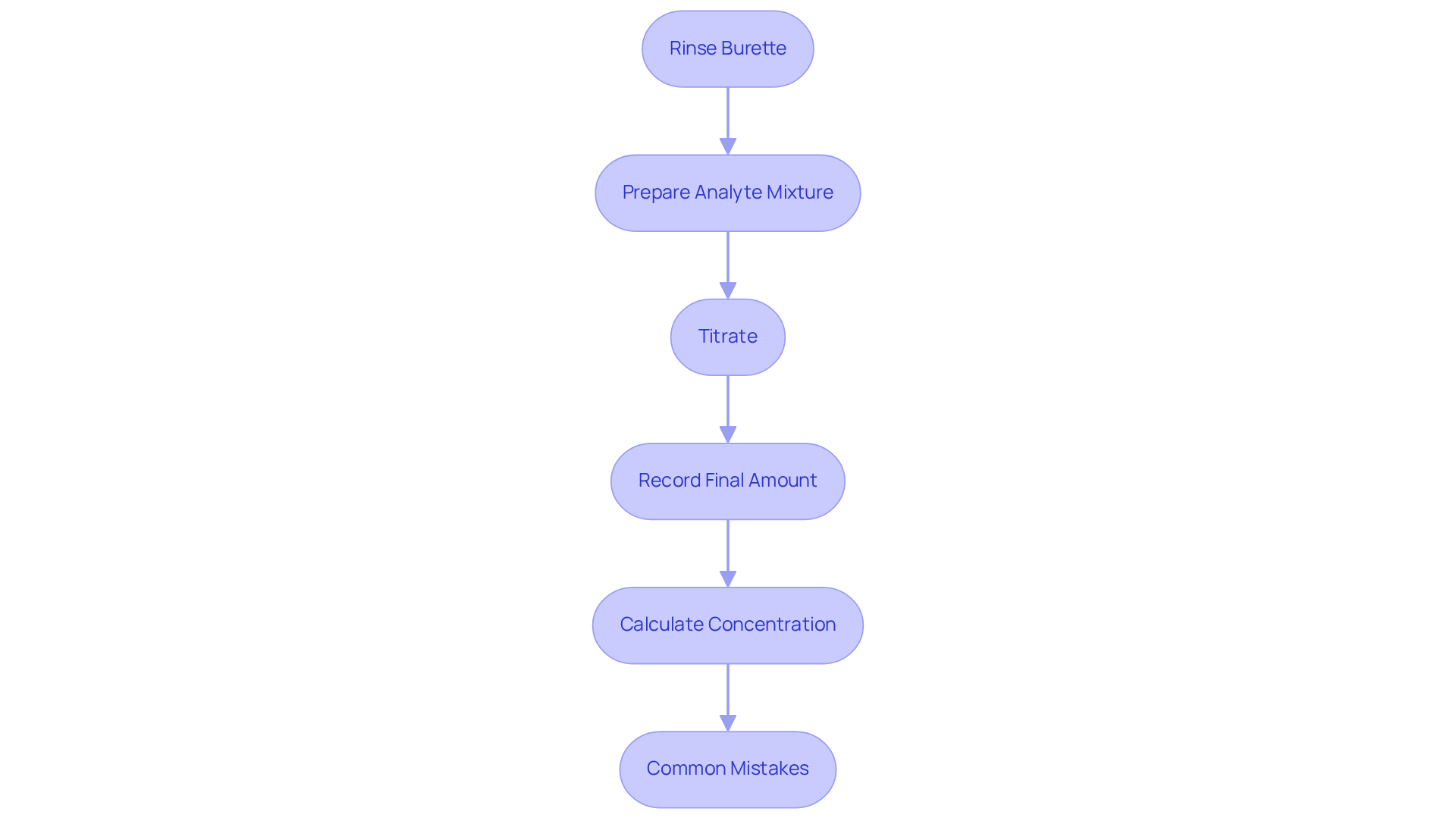
Follow Step-by-Step Titration Procedure
- In , you should begin by thoroughly rinsing the burette with distilled water, followed by rinsing it with the reagent to eliminate any potential contaminants. Fill the burette with the reagent and accurately record the starting volume.
- Prepare the Analyte Mixture: Employ a pipette to measure a precise volume of the analyte mixture, transferring it into an Erlenmeyer flask. If necessary, introduce a few drops of an appropriate indicator to aid in observing the endpoint.
- Titrate: Position the Erlenmeyer flask beneath the burette. In the redox titration lab, gradually add the reagent to the analyte solution while continuously swirling the flask to ensure thorough mixing. Watch for a color change that indicates the endpoint of the titration.
- Record the Final Amount: Once the endpoint is reached, document the final volume of the solution in the burette. Calculate the volume of solution used by subtracting the initial measurement from the final reading.
- Calculate Concentration: Utilize the formula to determine the concentration of the analyte by applying the volume of reagent consumed along with its known concentration.
Common Mistakes and How to Avoid Them:
- It is crucial to ensure that the burette is devoid of air bubbles, as these can result in inaccurate measurements.
- Additionally, avoid adding the titrant too rapidly, as this may lead to overshooting the endpoint.
- Systematic errors in the redox titration lab can consistently skew results due to flaws in the experimental setup, making careful calibration essential.
Real-World Examples:
- In pharmaceutical research, precise measurement is vital for determining the concentration of active ingredients in drug formulations.
- For instance, a study demonstrated that accurate measuring methods improved the precision of dosage calculations, directly impacting patient safety and treatment effectiveness.
- Titration curves provide a visual representation of pH variations throughout the procedure, illustrating the relationship between pH and titrant volume, which is critical for accurate analysis.
Expert Insights:
- Experienced chemists emphasize that "the selection of a suitable indicator is as crucial as the method itself," highlighting the importance of choosing indicators that provide clear visual signals at the equivalence point.
- Accurate measurements in the redox titration lab can significantly influence laboratory results, with studies indicating that even minor discrepancies can lead to substantial variations in outcomes.
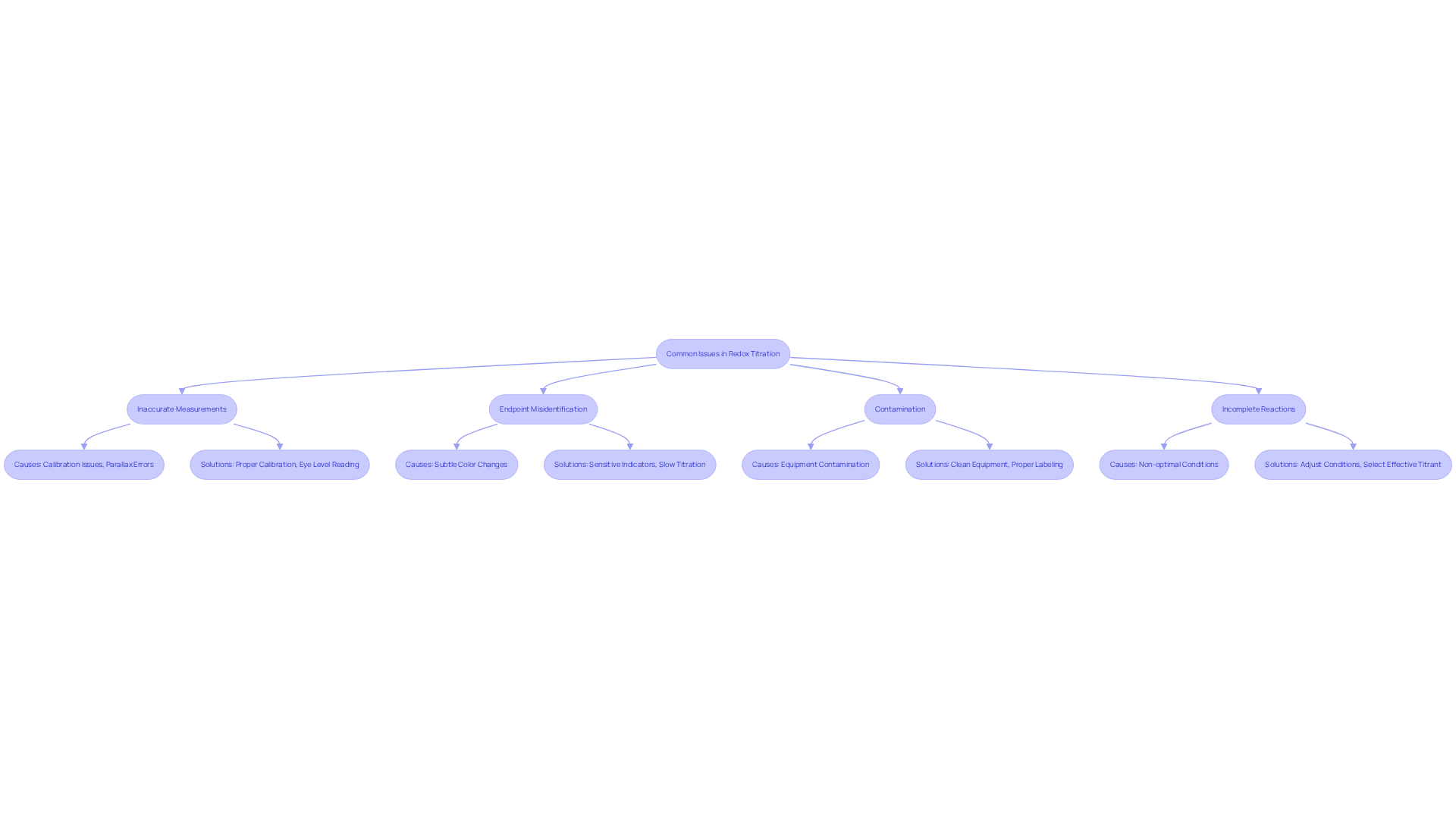
Troubleshoot Common Issues in Redox Titration
Common issues during a redox titration lab can significantly impact measurement accuracy. Understanding these challenges and their solutions is essential for achieving reliable results in a redox titration lab.
- Inaccurate Measurements: Calibration of glassware is crucial. Always read the burette at eye level to avoid parallax errors, which can lead to significant inaccuracies in volume measurement. Studies indicate that improper reading techniques contribute to a notable percentage of analytical errors in laboratory settings, with an overall frequency of errors reported at 3,092 parts per million (ppm). Ensuring accurate measurements in the redox titration lab is essential for maintaining the integrity of your results.
- Endpoint Misidentification: Subtle color changes can lead to misinterpretation of the endpoint. To mitigate this, consider using more sensitive indicators or titrating slowly as you approach the endpoint. This approach can help ensure that the endpoint is accurately identified, reducing the likelihood of over-titration. As noted by Laura K Snydman, stem from inadequate labeling processes, which can also affect endpoint determination. Addressing these factors is vital for achieving precision in the redox titration lab.
- Contamination: Contamination is a frequent issue that can skew results. Always wash your equipment with the substances they will hold to prevent cross-contamination. Utilizing clean pipettes and burettes for each solution is crucial to preserve the accuracy of your results. The importance of proper labeling and handling is emphasized in the context of preanalytical errors, which account for 61.9% of confirmed laboratory errors. Vigilance in contamination prevention is key to maintaining the reliability of your findings in the redox titration lab.
- Incomplete Reactions: Optimal reaction conditions are vital for complete reactions. Ensure that factors such as temperature and pH are conducive to the experiments conducted in the redox titration lab. If reactions are incomplete, consider adjusting these conditions or selecting a different titrant that may be more effective under the current circumstances. Implementing a Failure Mode and Effects Analysis (FMEA) can help identify potential failures in these processes and enhance overall laboratory safety. A proactive approach to reaction conditions can significantly improve your analytical outcomes.
By proactively addressing these common issues in your redox titration lab and incorporating systematic approaches like FMEA, you can enhance the accuracy and reliability of your titration results, ultimately leading to more dependable analytical outcomes.
Conclusion
Mastering the redox titration lab is essential for anyone engaged in analytical chemistry. This intricate process enables precise determination of an unknown solution's concentration while underscoring the critical importance of understanding oxidation-reduction reactions. By grasping the principles of redox titration, chemists can significantly enhance the accuracy of their measurements, a necessity in fields such as pharmaceuticals and quality control.
The article explores the foundational elements vital for successful redox titration, encompassing the necessary materials and equipment, a step-by-step procedure, and common troubleshooting techniques. Key points, such as:
- The significance of using calibrated glassware
- The careful selection of indicators
- The prevention of contamination
are highlighted as essential practices that ensure reliable results. Furthermore, addressing common issues like endpoint misidentification and incomplete reactions can further bolster the integrity of analytical outcomes.
Engaging with the redox titration process not only enriches laboratory skills but also contributes significantly to the broader applications of analytical chemistry. As the demand for precision in chemical analysis escalates, mastering these techniques becomes increasingly paramount. Embracing a proactive approach to troubleshooting and adhering to best practices will not only enhance individual performance in the lab but also elevate the standards of scientific inquiry and innovation within the field.




