Overview
Mastering temperature and pH is paramount for achieving accurate lab measurements. Both factors exert a significant influence on chemical reactions and biological processes. Notably, even minor deviations in temperature and pH can lead to substantial inaccuracies in experimental results. This underscores the necessity for precise monitoring and calibration to maintain optimal conditions, ensuring reliable scientific outcomes. In the realm of laboratory science, the importance of these elements cannot be overstated.
Introduction
Understanding the intricate balance of temperature and pH is essential for achieving reliable results in laboratory experiments. These two fundamental parameters not only influence the efficacy of biochemical reactions but also determine the overall success of scientific inquiries. How can researchers ensure that they consistently measure and adjust these variables to maintain optimal conditions? This article explores the essential practices for mastering temperature and pH, emphasizing their significance in laboratory accuracy and addressing common challenges that may arise in the process.
Understand Temperature and pH Basics
Temperature quantifies the average kinetic energy of particles in a substance, typically expressed in degrees Celsius (°C) or Kelvin (K). pH, conversely, is a logarithmic scale indicating the acidity or basicity of an aqueous solution, ranging from 0 (highly acidic) to 14 (highly basic), with 7 representing neutrality. Understanding these definitions is crucial; both temperature and pH levels substantially affect chemical reactions and biological processes in laboratory settings.
For instance, studies show that variations in heat of merely 3 °C can modify substrate affinity and enzymatic reaction rates, highlighting the importance of accurate measurements. Moreover, research has demonstrated that sustaining ideal pH levels is essential for the stability of proteins and nucleic acids, as unregulated thermal conditions can result in denaturation. Consequently, precise observation of both temperature and pH is essential for guaranteeing dependable experimental results and promoting research in diverse scientific areas.
Recognize the Importance of Optimal Conditions
Obtaining precise and uniform outcomes in laboratory experiments is paramount, hinging on the maintenance of ideal conditions for temperature and pH. Numerous biochemical processes, particularly those involving human catalysts, operate optimally at approximately 37°C. For instance, proteins like lactate dehydrogenase (LDH) exhibit peak activity at this temperature, whereas thermophilic proteins such as Taq polymerase thrive at elevated temperatures ranging from 75 to 80°C. Additionally, DNA ligase functions best at around 25°C.
The functionality of proteins is critically dependent on both temperature and pH levels; each protein has a specific pH range that maximizes its effectiveness. Deviations from these optimal conditions can lead to significant inaccuracies, misinterpretations, and wasted resources. For example, employing catalysts at temperatures below their ideal range can result in low yields of desired products, while excessive heat can cause denaturation, permanently impairing catalyst activity.
Laboratory managers have noted that even minor fluctuations of 1 or 2 degrees can alter results by 10% to 20%. As Dr. Nick Oswald aptly states, "If you accidentally use a setting that is too high, you risk denaturing the enzymes and losing their desired activity." Therefore, regular monitoring and adjustment of the temperature and pH parameters are essential to uphold the integrity of laboratory work and ensure reliable experimental outcomes.
Measure and Adjust Temperature and pH Levels
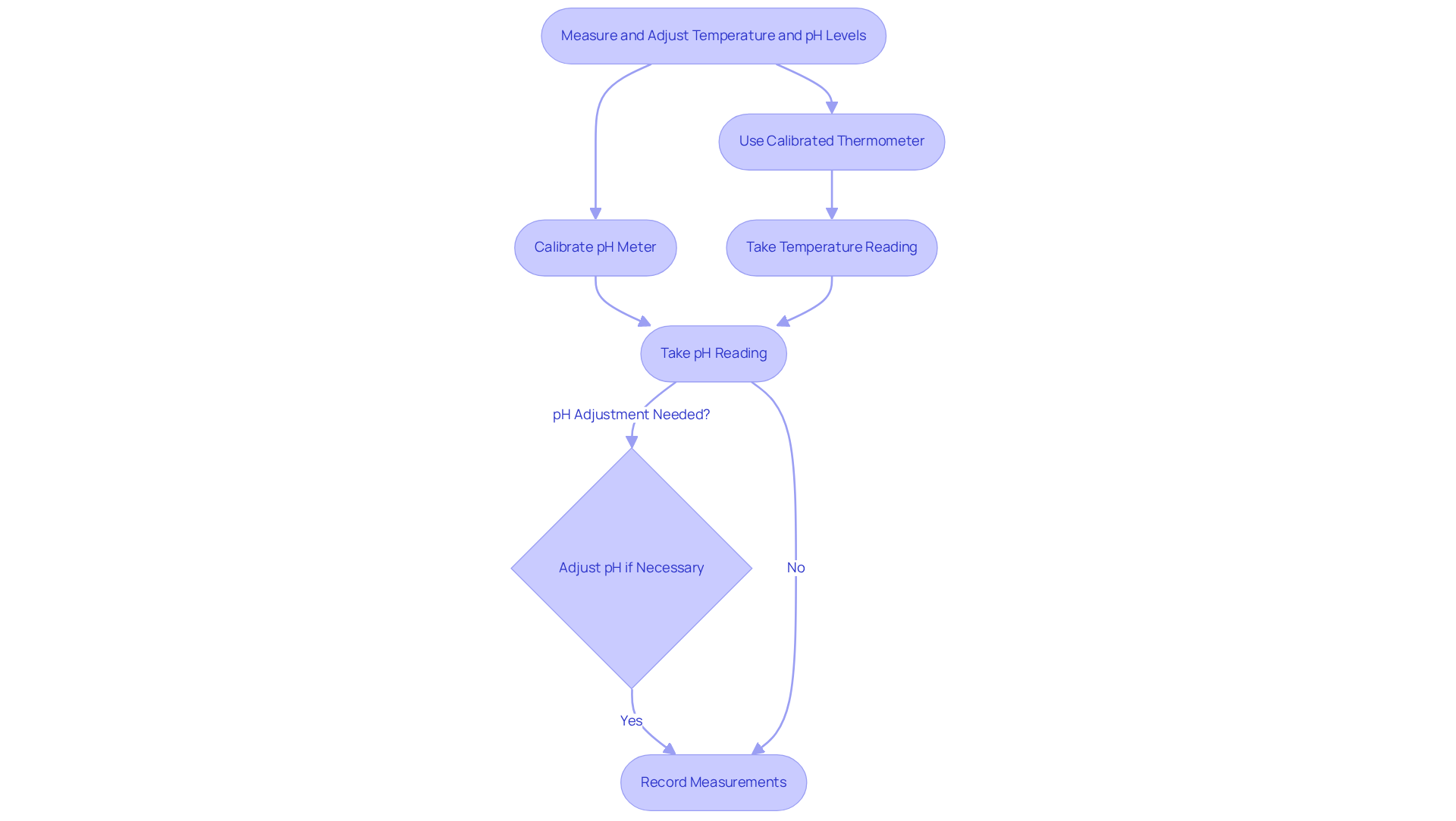
To assess warmth accurately, it is essential to utilize a calibrated thermometer tailored for your specific application. For instance, a digital thermometer is ideal for rapid readings, while a mercury thermometer is preferable for precise evaluations. Ensure that the thermometer is fully submerged in the solution to achieve adequate stabilization before recording the reading.
When evaluating pH, a calibrated pH meter is necessary. Begin by calibrating the meter with certified standard buffer solutions, specifically pH 4 and pH 7, to guarantee accuracy. After calibration, immerse the electrode in the solution and allow it to stabilize before taking a reading. Should adjustments be required, gradually add acids or bases to the solution, closely monitoring the pH until the desired level is achieved.
Consistently record the temperature and pH measurements, while maintaining a calibration logbook for traceability and adherence to standards. Regular calibration is crucial; internal calibrations should occur at least once daily, while external calibrations should be performed annually by authorized service providers to ensure ongoing accuracy. The offset value during calibration must not exceed ±20 mV, and the slope value should remain within the range of 95% to 105%.
By adhering to these best practices, you not only enhance the reliability of assessments but also facilitate compliance with Good Documentation Practices (GDocP), which are essential for quality control in testing environments. Furthermore, be aware that fluctuations in temperature and pH can influence readings, making it vital to consider both temperature and pH in your laboratory procedures.
Troubleshoot Common Temperature and pH Issues
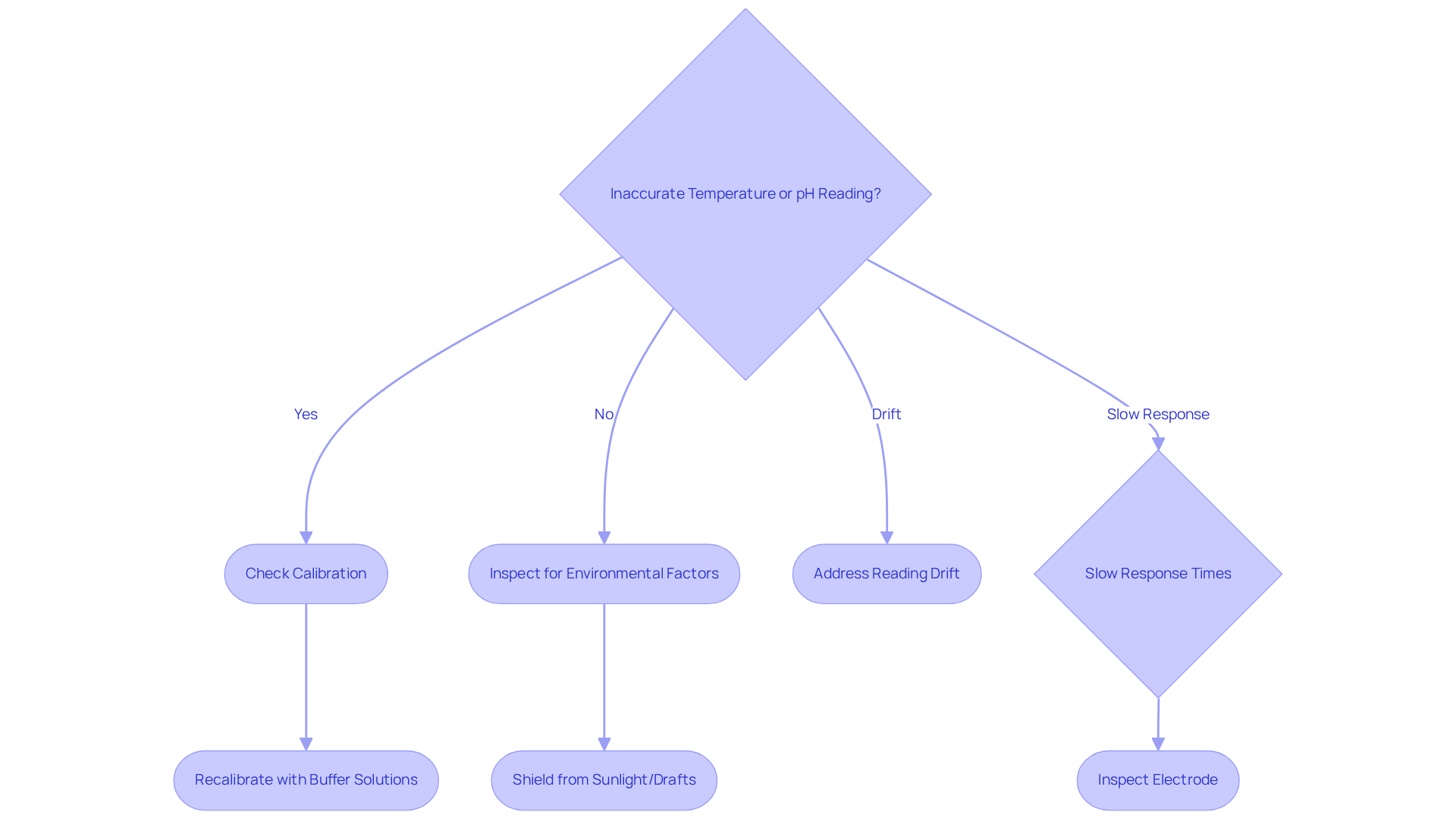
Inaccurate readings of temperature and pH often arise from improper calibration or environmental factors, such as direct sunlight exposure or drafts. To mitigate these issues, it is essential to frequently adjust the thermometers for temperature and pH and ensure they are shielded from external influences during evaluations.
For pH meters, common challenges include:
- Reading drift
- Slow response times
When a pH meter exhibits drift, recalibrating with fresh buffer solutions is advisable. Slow response times may signal contamination or damage to the electrode, necessitating a thorough inspection. Regular maintenance of the pH electrode, which includes cleaning and proper storage, is vital for ensuring precise readings.
Statistics indicate that calibration intervals for pH sensors can vary significantly, ranging from daily to monthly, depending on the specific conditions of the facility and the performance requirements of the sensor. Laboratory professionals frequently report that ongoing issues with pH meters often necessitate electrode replacement, particularly when the electrode has reached the end of its operational lifespan. By following a proactive maintenance schedule and addressing common issues promptly, laboratories can improve the reliability and accuracy of their pH measurements.
Conclusion
Mastering temperature and pH is essential for achieving accurate and reliable laboratory measurements. These two critical parameters profoundly influence chemical reactions and biological processes, underscoring the necessity for precise monitoring and control. A comprehensive understanding of temperature and pH not only enhances the validity of experimental results but also promotes efficiency in research and development across various scientific fields.
This article outlines the fundamental concepts of temperature and pH, emphasizing their roles in biochemical processes and the optimal conditions required for specific reactions. Key insights include:
- The importance of maintaining ideal temperature ranges for different proteins
- The meticulous procedures needed for measuring and adjusting pH levels
Regular calibration and proactive troubleshooting are vital to mitigate common issues, ensuring that laboratories uphold the highest standards of accuracy and reliability.
Ultimately, the significance of mastering temperature and pH in laboratory settings cannot be overstated. By prioritizing these aspects, researchers can enhance the integrity of their experiments, minimize errors, and contribute to the advancement of scientific knowledge. Implementing best practices in measurement and adjustment will yield better results and foster a culture of precision and excellence in laboratory work.




