Overview
Mastering the analyte in titration requires several essential steps that are crucial for achieving reliable results.
- First, it is imperative to understand the fundamental principles of titration.
- Next, gathering the necessary equipment is vital to ensure that the process runs smoothly.
- Following a precise step-by-step procedure is equally important, as it minimizes errors and enhances accuracy.
- Additionally, troubleshooting common issues that may arise during the titration process is essential for maintaining the integrity of the results.
This article underscores the significance of accurate measurements and proper equipment use. It highlights how careful observation during the titration process is paramount to achieving dependable outcomes. By adhering to these guidelines, one can ensure that the titration results are not only valid but also reproducible, reinforcing the importance of high-quality scientific instruments in laboratory settings.
Introduction
Mastering the art of titration is essential for anyone engaged in quantitative analysis; it serves as a critical method for determining the concentration of unknown solutions. This guide outlines the fundamental principles and necessary materials while providing a step-by-step approach to performing titrations with precision. Even seasoned chemists can encounter challenges that may compromise their results.
What are the common pitfalls in the titration process?
How can they be effectively navigated to ensure accurate measurements?
Understanding these aspects is vital for achieving reliable outcomes in laboratory settings.
Understand the Basics of Titration
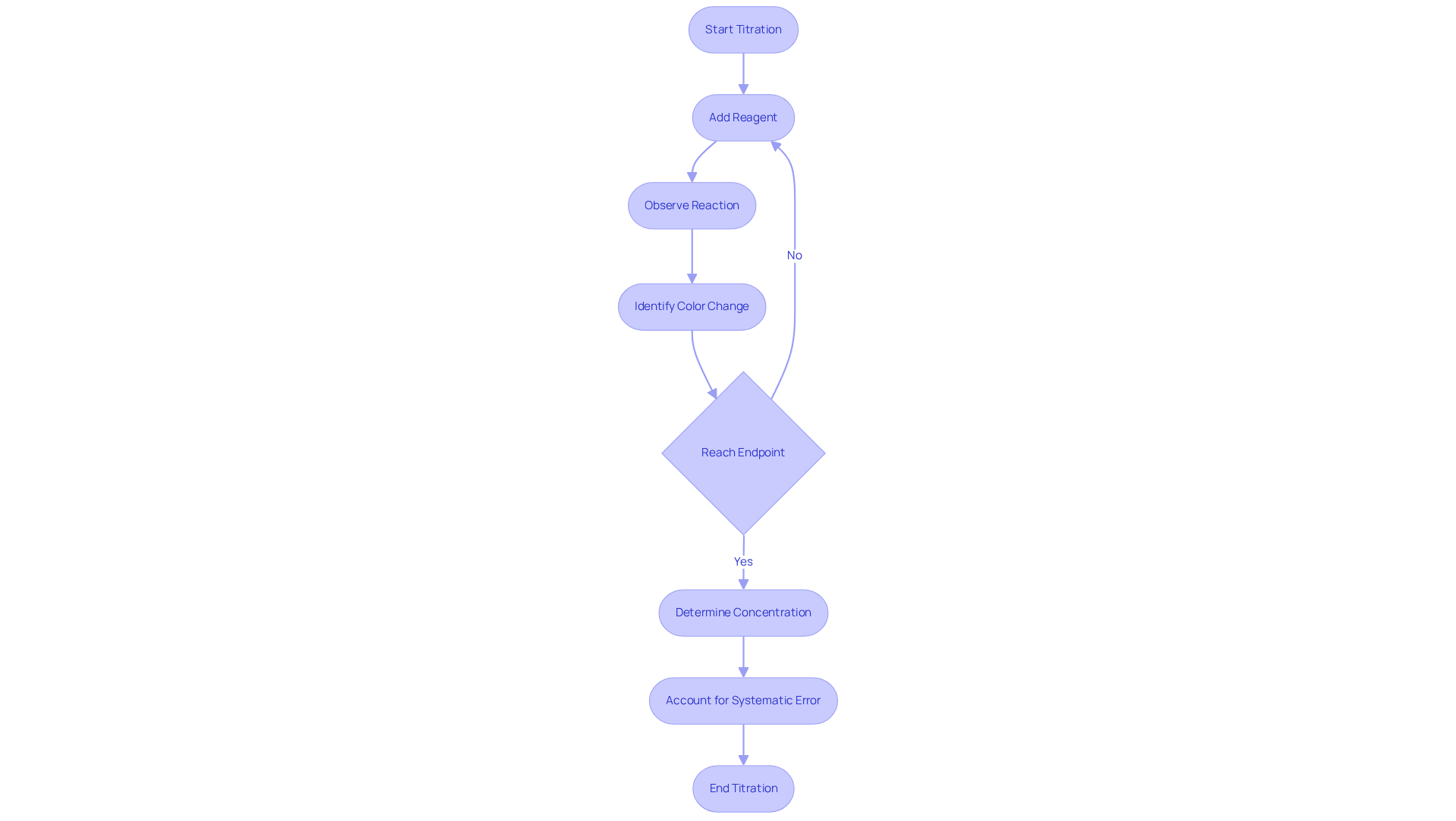
Titration serves as a fundamental , crucial for determining the concentration of an unknown solution, known as the analyte, by reacting it with a reagent of known concentration. This method involves the meticulous addition of the reagent to the sample until the reaction reaches its conclusion, typically indicated by a notable color change from an added indicator.
A solid grasp of the concepts of equivalence point and endpoint is essential for effective quantitative analysis. The equivalence point, where the amount of titrant precisely matches the amount of analyte, is critical for accurately determining the analyte's concentration. Understanding these terms not only clarifies the process but also enhances the precision of result interpretation.
In practical laboratory applications, such as pharmaceuticals and environmental testing, mastery of these concepts ensures reliable measurements and plays a vital role in quality assurance for product formulation and adherence to regulatory standards.
The Lab Titration Devices Market is projected to grow from USD 1.95 billion in 2025 to USD 2.67 billion by 2030, underscoring the increasing significance of these techniques within the pharmaceutical industry. As Tracy Costello, PharmD, BCPS, states, "Glycemic management through intravenous insulin infusions is a vital aspect of hyperglycemic crisis care," highlighting the crucial role of precise dosage adjustments in clinical settings.
Moreover, understanding systematic error—defined as the difference between the volume of reagent added to reach the endpoint and the stoichiometric equivalent point—is vital for ensuring measurement accuracy.
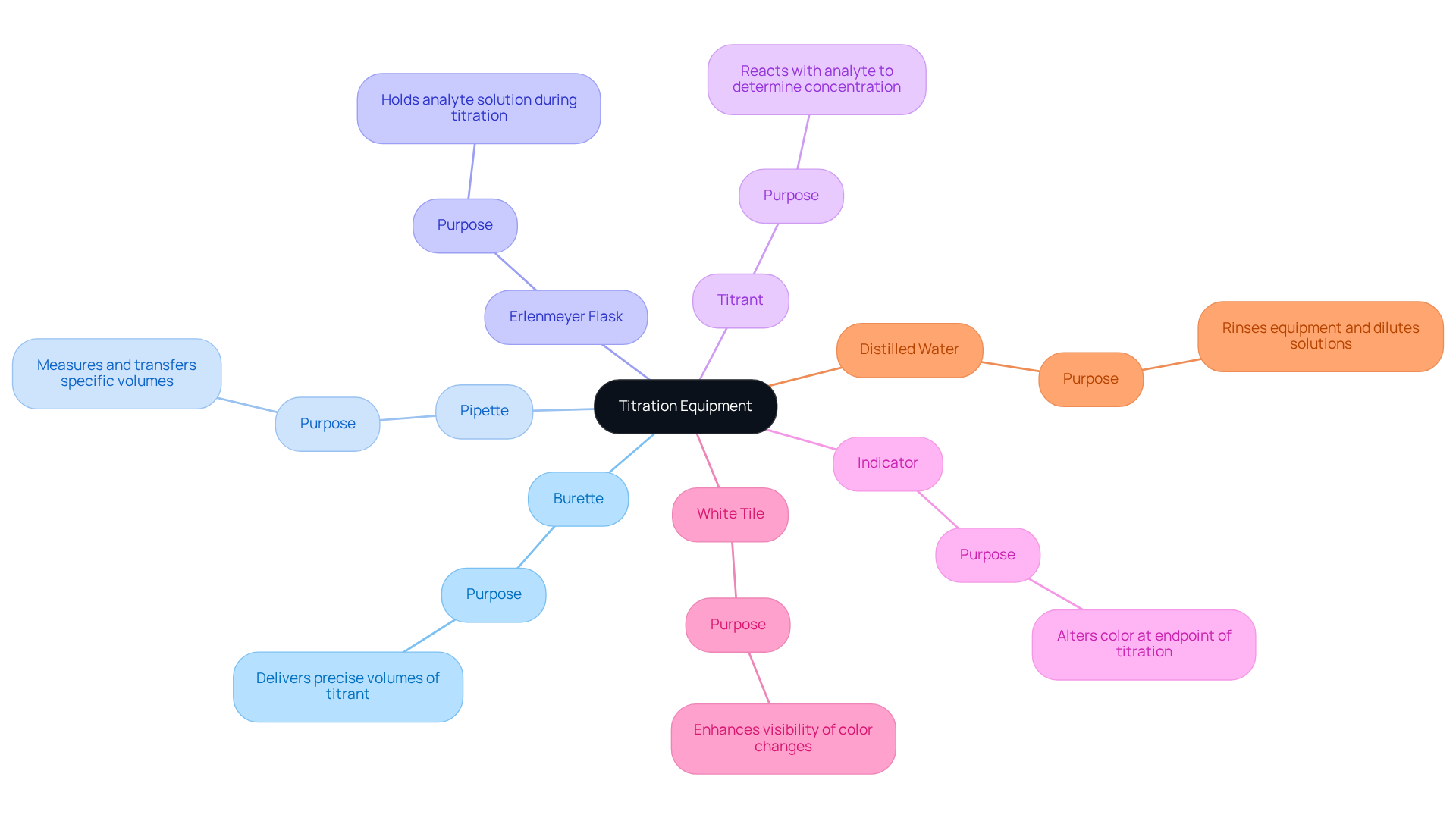
Gather Necessary Materials and Equipment
To successfully , it is essential to gather specific materials and equipment that ensure precision and accuracy in your results. The following items are necessary:
- Burette: A graduated glass tube that allows for the delivery of precise volumes of titrant, which is critical for accurate measurements.
- Pipette: Used for measuring and transferring a specific volume of the substance, ensuring that you maintain consistency in your analysis.
- Erlenmeyer flask: Serves as the vessel to hold the analyte solution during the titration process, providing stability and ease of mixing.
- Titrant: A solution of known concentration—typically an acid or base—that is fundamental to the titration process, as it reacts with the analyte to determine its concentration.
- Indicator: A chemical that alters color at the endpoint of the titration, with phenolphthalein being a common choice.
- White tile: Recommended to enhance visibility of color changes, allowing for clearer observation of the reaction.
- Distilled water: Vital for rinsing equipment and diluting solutions when necessary, ensuring that all materials are free from contaminants.
Before commencing the titration, it is imperative to ensure that all equipment is clean and calibrated. This preparation is crucial to avoid contamination and inaccuracies, which could compromise the integrity of your results. By adhering to these guidelines, you set the stage for a successful titration that yields reliable and precise data.
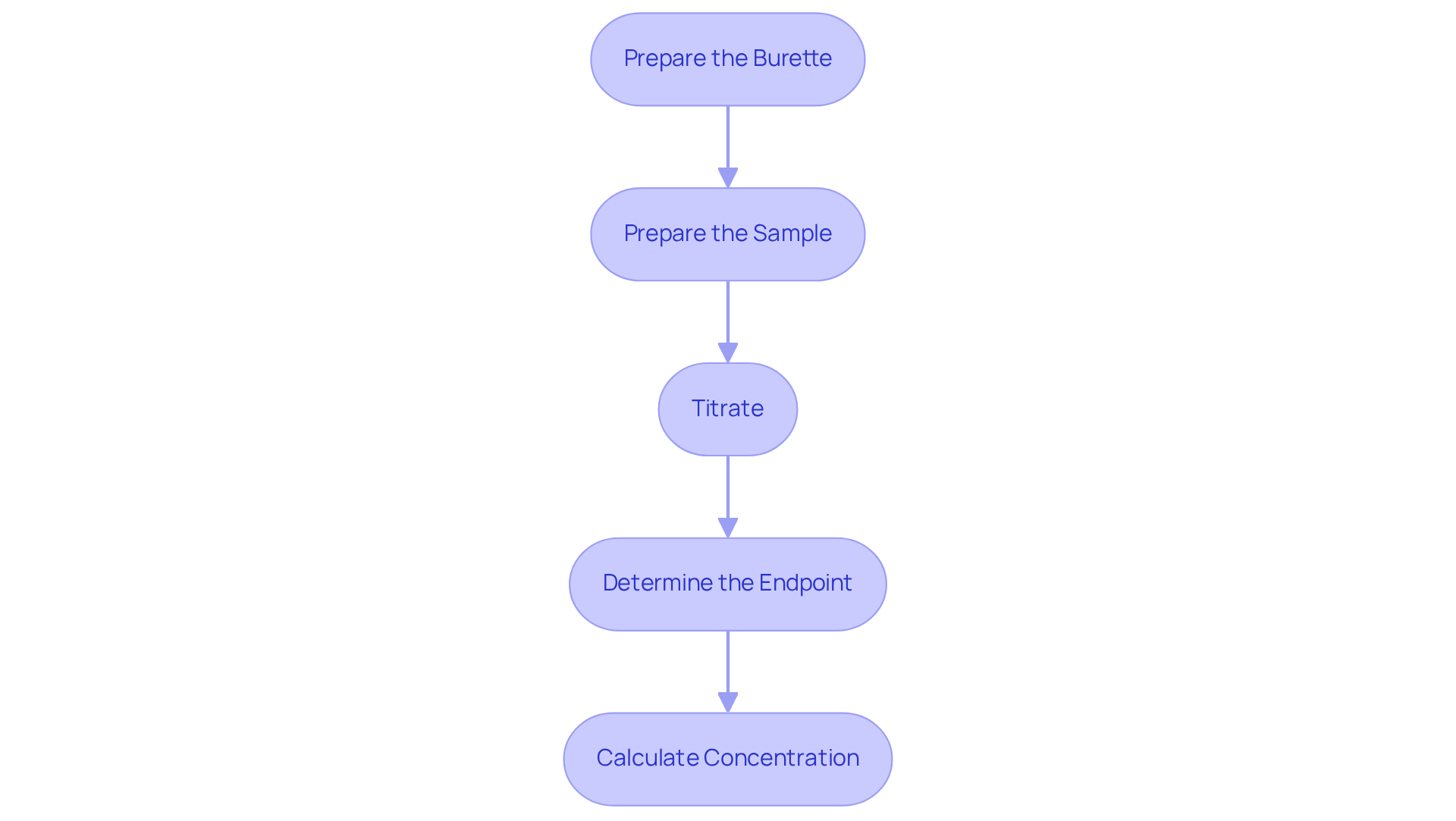
Follow the Step-by-Step Titration Procedure
-
Prepare the Burette: Begin by rinsing the burette thoroughly with distilled water, followed by rinsing it with the solution to prevent contamination. Fill the burette with the titrant and accurately record the initial volume to ensure precise measurements.
-
Prepare the Sample: Employ a pipette to measure a precise volume of the sample solution, transferring it into an Erlenmeyer flask. Introduce a few drops of the selected indicator to the flask, which will assist in detecting the endpoint of the titration.
-
Titrate: Place the Erlenmeyer flask on a white tile to enhance the visibility of color changes during the titration process. Gradually introduce the reagent from the burette to the analyte while continuously swirling the flask. Monitor the solution for a color change, which indicates that you are approaching the endpoint.
-
Determine the Endpoint: As you near the endpoint, add the reagent dropwise until the color change remains consistent for at least 30 seconds. Record the final volume of the solution in the burette to facilitate accurate calculations.
-
Calculate Concentration: To ascertain the concentration of the analyte, utilize the volume of titrant consumed along with its concentration in the following formula:
C1V1 = C2V2
In this equation, C1 and V1 represent the concentration and volume of the titrant, while C2 and V2 denote the concentration and volume of the analyte. This calculation is vital for of your results.
Troubleshoot Common Titration Issues
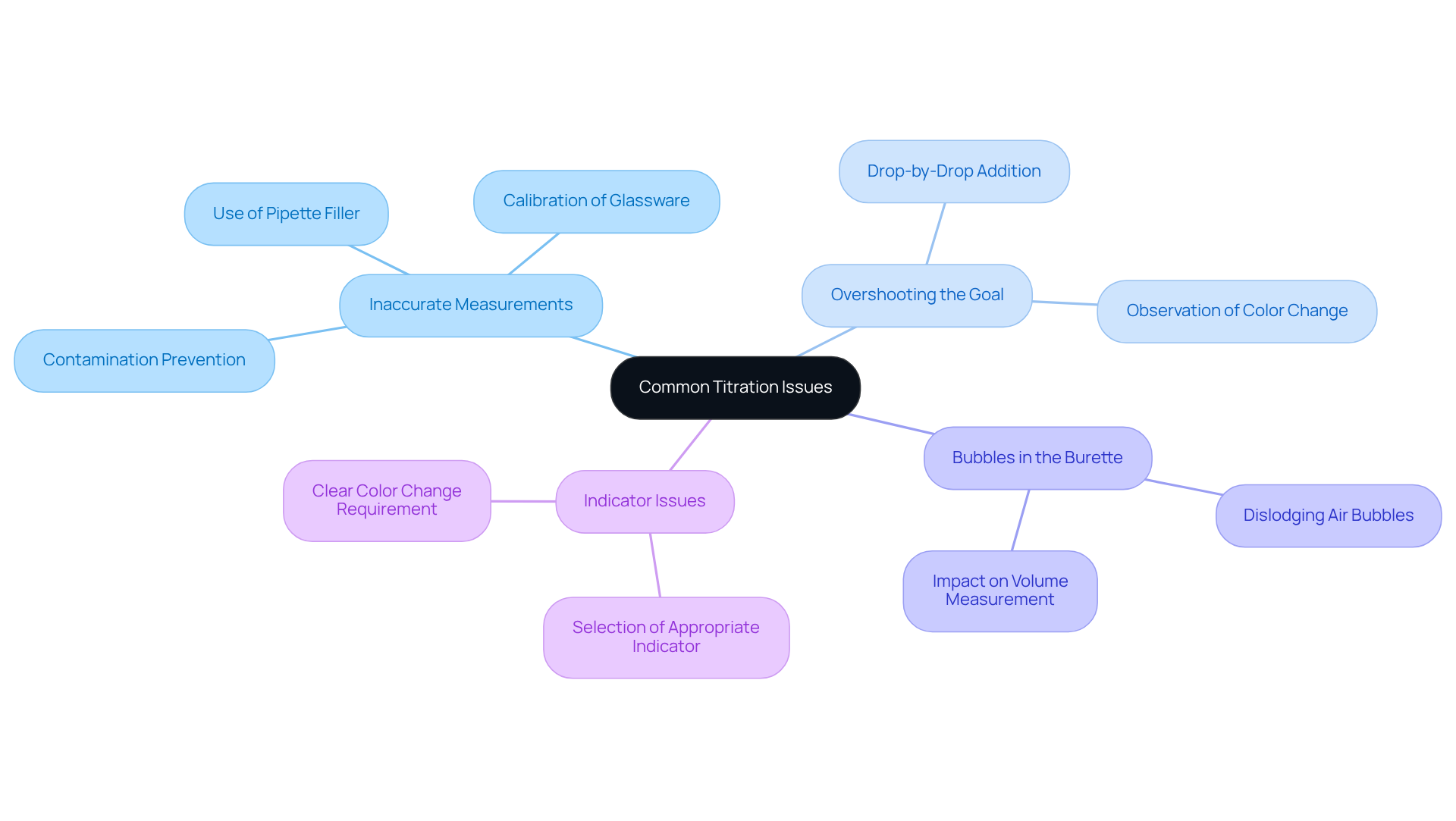
Common issues encountered during titration include:
- Inaccurate Measurements: Precision is paramount in titration. To achieve this, all glassware must be calibrated and meticulously cleaned. Utilizing a pipette filler is essential to when measuring the analyte; even minor impurities can skew results. According to the American Chemical Society, improper calibration can lead to measurement errors that significantly affect the outcome of titrations.
- Overshooting the Goal: As you approach the conclusion of the titration, adding the reagent drop by drop is crucial. This careful approach helps prevent overshooting, which can lead to substantial inaccuracies. Attentively observing the color change is vital for accurately determining the endpoint. As emphasized by Metrohm's Sr. Product Specialist, careful observation and controlled addition of titrant are key to achieving reliable results.
- Bubbles in the Burette: Air bubbles can result in incorrect volume measurements, adversely affecting accuracy. Gently tapping the burette can dislodge any trapped air before starting the process, ensuring a more reliable measurement. A study indicated that air bubbles can introduce a tolerance error of ±0.05 mL in 50 mL burets, which can significantly affect the final results.
- Indicator Issues: The selection of an appropriate indicator is essential for successful analysis. Some indicators may fail to exhibit a clear color change for specific reactions, leading to confusion. It is crucial to select a measure that aligns with the type of analysis to aid in precise endpoint identification. As noted by renowned chemist Robert Bunsen, "The choice of the indicator is as important as the choice of the titrant."
In instances where issues persist, conducting an initial measurement can provide a more precise estimation of the endpoint before executing the actual analysis. This practice not only enhances accuracy but also builds confidence in the results. A case study on best practices for accurate results highlights that preliminary titrations can significantly reduce the likelihood of errors in final analyses.
Conclusion
Mastering the analyte in titration is essential for achieving accurate and reliable results in quantitative analysis. Understanding the fundamental principles of titration, along with the importance of precise measurements, enables one to effectively determine the concentration of an unknown solution. This mastery not only enhances the quality of laboratory results but also plays a pivotal role in various industries, particularly in pharmaceuticals and environmental testing.
Throughout this article, we have outlined essential steps such as:
- Preparing the necessary materials
- Adhering to a structured procedure
- Troubleshooting common issues
Key equipment, including burettes, pipettes, and indicators, has been emphasized for their roles in facilitating accurate titration. Additionally, recognizing and addressing potential problems, such as inaccurate measurements or indicator selection, is vital for maintaining the integrity of the titration process.
As the significance of titration continues to grow across various applications, it is imperative for practitioners to refine their skills and knowledge. Engaging with the nuances of this analytical method will bolster confidence in laboratory settings and contribute to advancements in scientific research and quality assurance. Embracing these best practices will lead to more accurate results and a deeper understanding of the chemical principles at play, ultimately enhancing the overall effectiveness of titration in analytical chemistry.




