Overview
The Karl Fischer titration principle stands as a cornerstone in the precise measurement of moisture levels across various materials. This method hinges on the reaction between water and iodine in the presence of a base, showcasing its fundamental role in analytical chemistry. Notably, its effectiveness has earned it the status of an industry standard, underpinned by remarkable accuracy and selectivity for water. Furthermore, its versatility allows it to be applied to a wide range of materials, making it indispensable in laboratory settings.
However, it is crucial to acknowledge the method's limitations and the preparation requirements necessary for achieving optimal results. Understanding these factors not only enhances the reliability of the titration process but also ensures that the results obtained are both accurate and reproducible. By mastering the Karl Fischer titration principle, professionals can significantly improve their moisture analysis capabilities, thereby reinforcing their expertise in the field.
Introduction
Understanding the intricacies of moisture analysis is crucial for industries where precision is paramount, such as pharmaceuticals and food production. The Karl Fischer titration principle stands out as a leading method, renowned for its accuracy in measuring water content through a well-defined chemical reaction. However, advancements in technology and varying methodologies—volumetric versus coulometric—present laboratory professionals with the challenge of selecting the right approach for their specific needs. Key factors determine the success of this vital analytical technique, and navigating these complexities is essential to ensure reliable results.
Explore the Fundamentals of Karl Fischer Titration
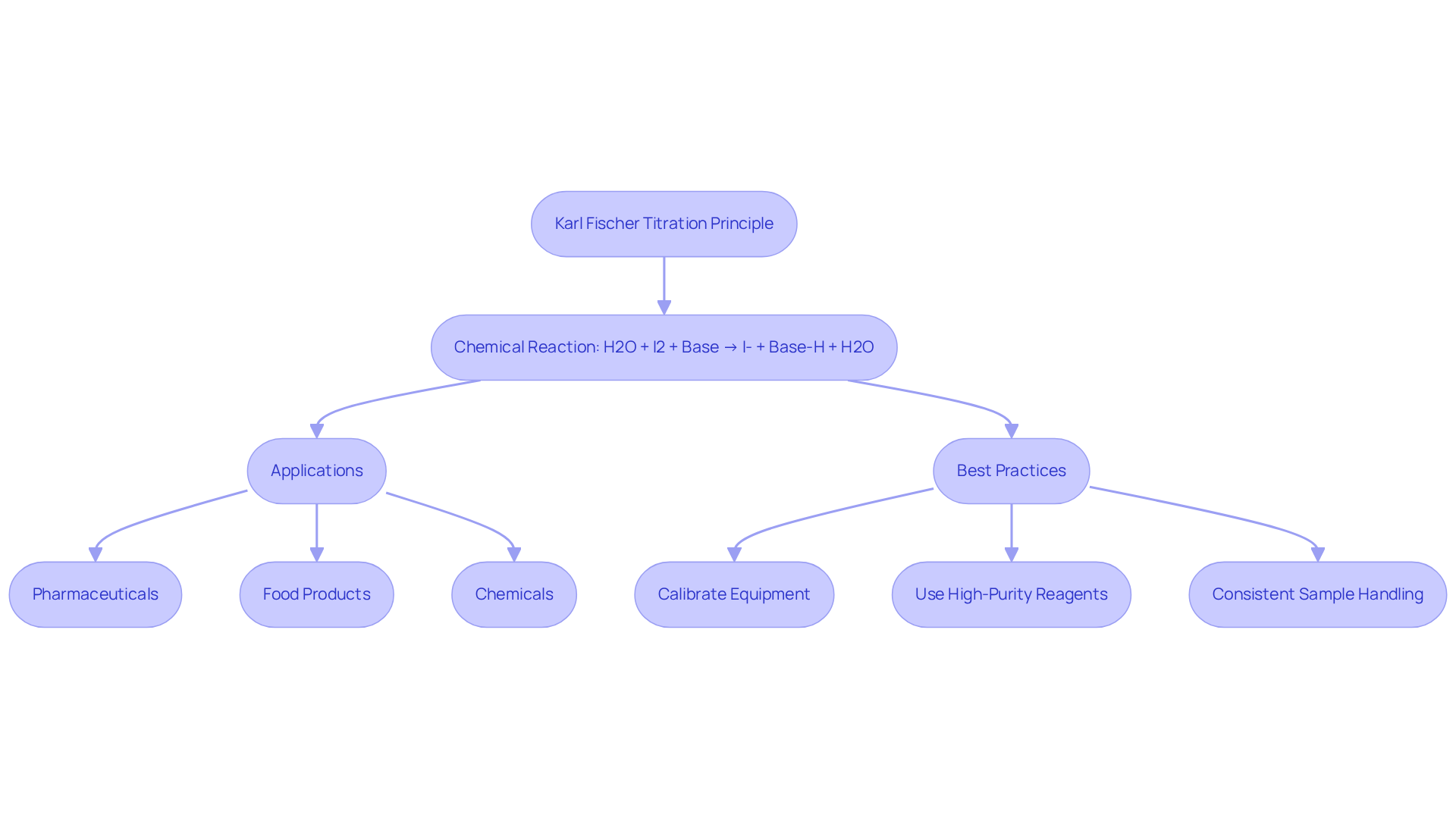
This analytical method is based on the Karl Fischer titration principle and stands as a premier technique for accurately assessing moisture levels across various materials. The Karl Fischer titration principle is based on the reaction of water with iodine in the presence of a base, typically pyridine or imidazole. The reaction can be succinctly summarized as follows:
H2O + I2 + Base → I- + Base-H + H2O
This reaction demonstrates exceptional selectivity for water, rendering it particularly effective for moisture analysis in industries such as pharmaceuticals, food products, and chemicals. The stoichiometry of the reaction is critical for understanding the Karl Fischer titration principle, enabling precise calculations of moisture content based on the iodine utilized during the analysis process.
Recent advancements in moisture analysis techniques have significantly enhanced the efficiency and accuracy of this process. For example, the advent of automated titrators has streamlined laboratory workflows, minimizing human error and increasing throughput. Currently, approximately 62.1% of laboratories engaged in moisture analysis employ the Karl Fischer titration principle, underscoring its status as the industry standard.
In practical applications, optimal procedures for this method include:
- Ensuring proper calibration of equipment
- Utilizing high-purity reagents
- Adhering to consistent sample handling protocols
These practices not only bolster measurement accuracy but also enhance the reliability of results across diverse applications. As the demand for precise moisture regulation intensifies, the relevance of this method in laboratory settings remains paramount. The Hiranuma Aquacounter AQV-300 Volumetric and AQ-300 Coulometric titrators are meticulously designed to meet the stringent standards of drug and medicine testing as outlined in the Japanese Pharmacopoeia. Understanding the capabilities of these advanced titrators, coupled with a foundational knowledge of the reaction, lays the groundwork for effective implementation of the Karl Fischer titration principle in laboratory practices.
Differentiate Between Volumetric and Coulometric Titration Methods
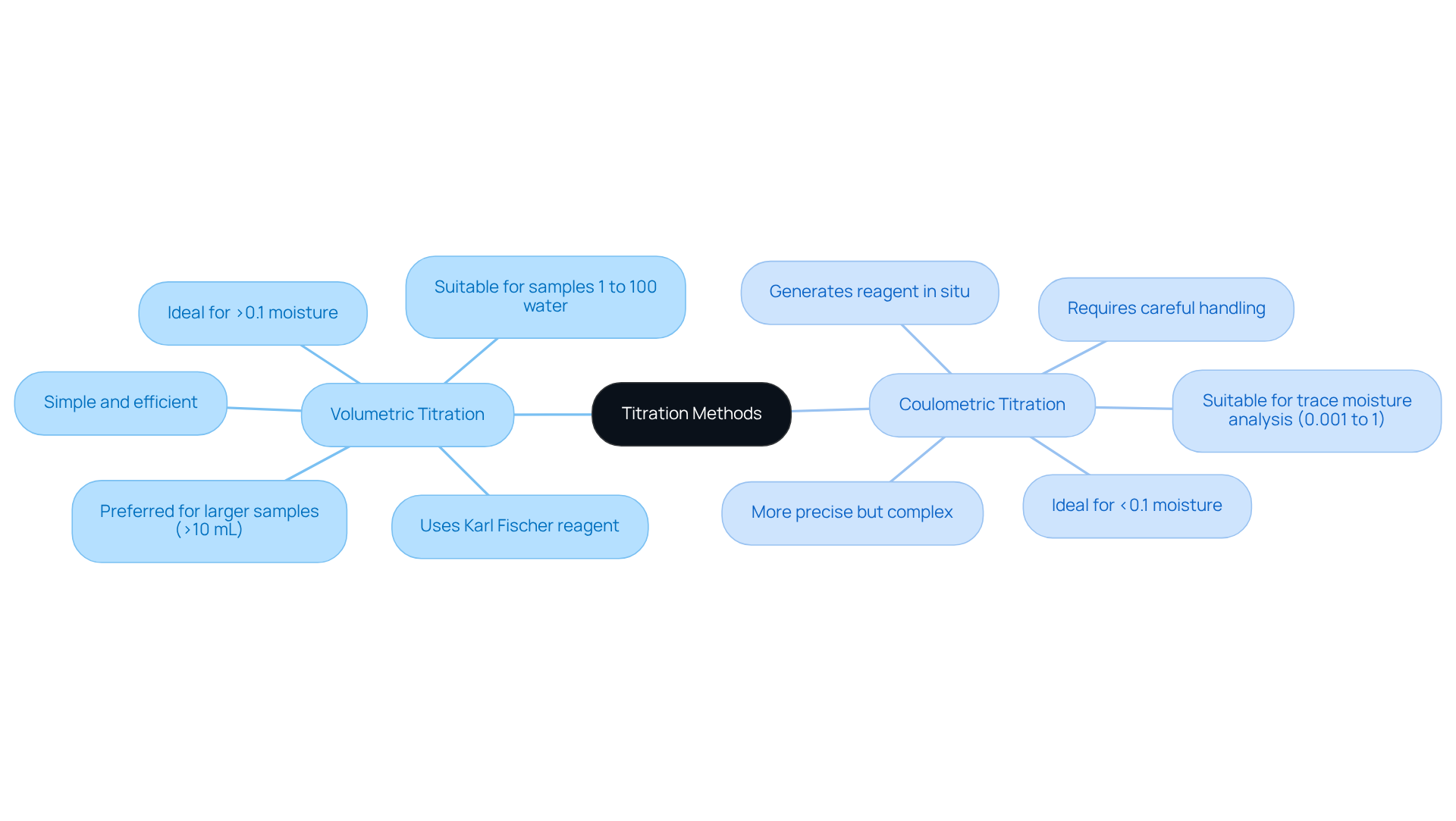
The titration technique is essential in laboratory analysis and can be conducted using two primary approaches: volumetric and coulometric.
-
Volumetric Titration operates on the Karl Fischer titration principle, which involves the addition of a known volume of Karl Fischer reagent to the sample until the endpoint is reached, indicated by a distinct color change. This method is particularly suitable for specimens with higher moisture content, typically exceeding 0.1%. Its simplicity and efficiency allow for the rapid analysis of multiple specimens, making it a preferred choice in many laboratories.
-
Coulometric Titration, on the other hand, generates the Karl Fischer titration principle reagent in situ through electrolysis. This method is ideal for specimens with low moisture content, specifically those below 0.1%, and it offers enhanced sensitivity. The endpoint is determined by measuring the current, which adds complexity but also precision to the analysis.
Understanding these distinctions is crucial for laboratory professionals. By selecting the appropriate method based on the moisture level of their materials, they can ensure accurate and reliable outcomes in their analyses.
Prepare for Karl Fischer Titration: Sample Requirements and Equipment Setup
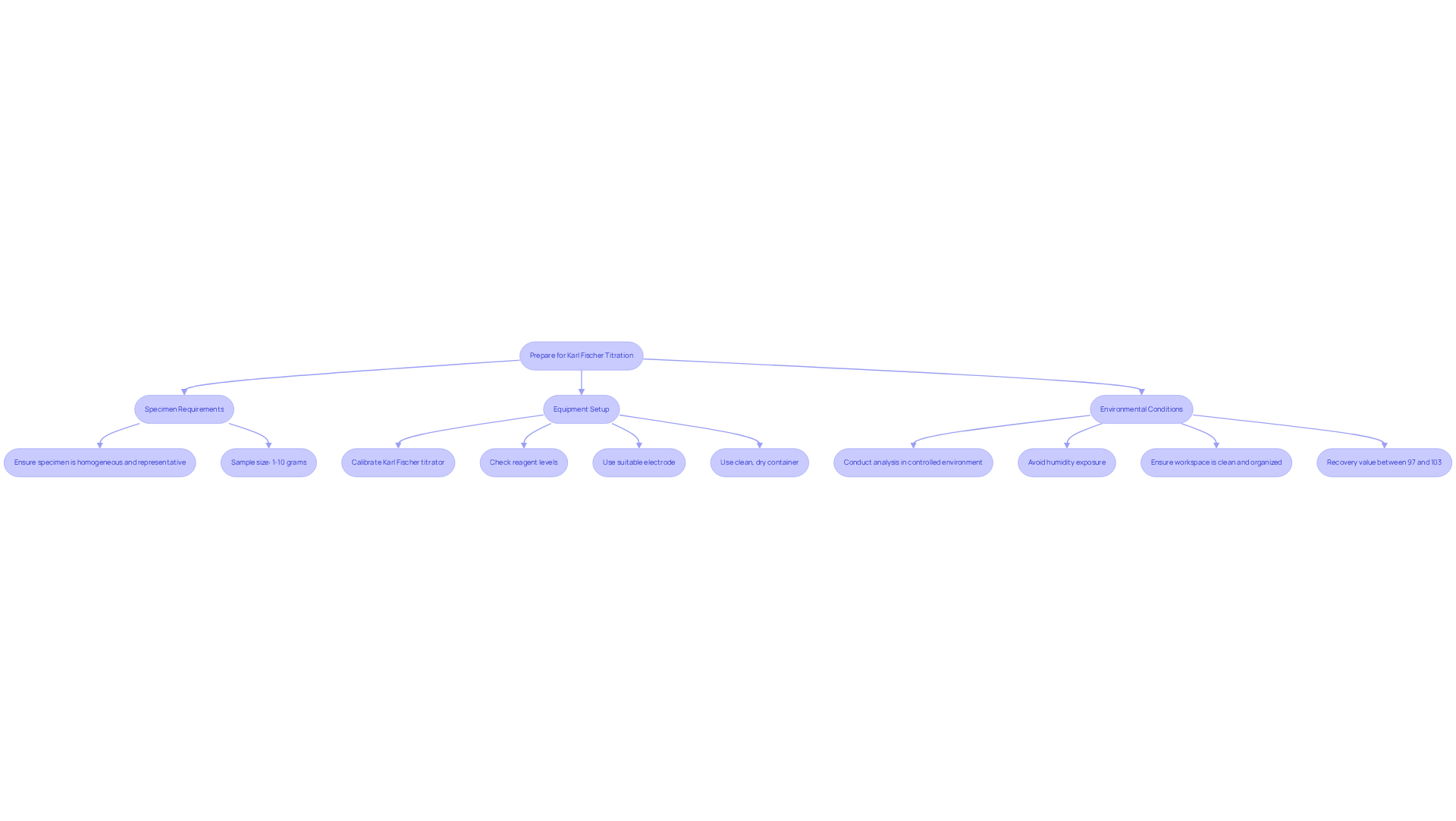
To successfully perform the Karl Fischer titration principle, proper preparation is essential. Key steps include the following:
-
Specimen Requirements: Ensure that the specimen is homogeneous and representative of the batch. The sample size should be appropriate for the expected moisture content, typically ranging from 1 to 10 grams, depending on the method used.
-
Equipment Setup:
- Titrator: Ensure that the Karl Fischer titrator is calibrated and functioning correctly. Check the reagent levels and replace any depleted reagents to maintain accuracy.
- Electrode: Use a suitable electrode for the selected method. For volumetric titration, a standard glass electrode is sufficient, while coulometric titration may require a specific type of electrode.
- Container: Use a clean, dry container to avoid contamination. Ensure that the vessel is compatible with the titrator. As noted by Michael Margreth, according to the Karl Fischer titration principle, in hermetically sealed vials, the contents are placed in the oven, and then water can be determined with any volumetric or coulometric KF titrator.
-
Environmental Conditions: Conduct the analysis in a controlled environment to minimize moisture interference. Avoid exposure to humidity and ensure that the workspace is clean and organized. The oven method is particularly effective for samples that release water slowly or require high temperatures for analysis, ensuring optimal performance in moisture determination.
By adhering to these preparation steps, laboratory professionals can enhance the precision and reliability of their results. It is crucial to note that the recovery value should be between 97% and 103% for proper system performance, underscoring the importance of meticulous preparation.
Evaluate the Advantages and Limitations of Karl Fischer Titration
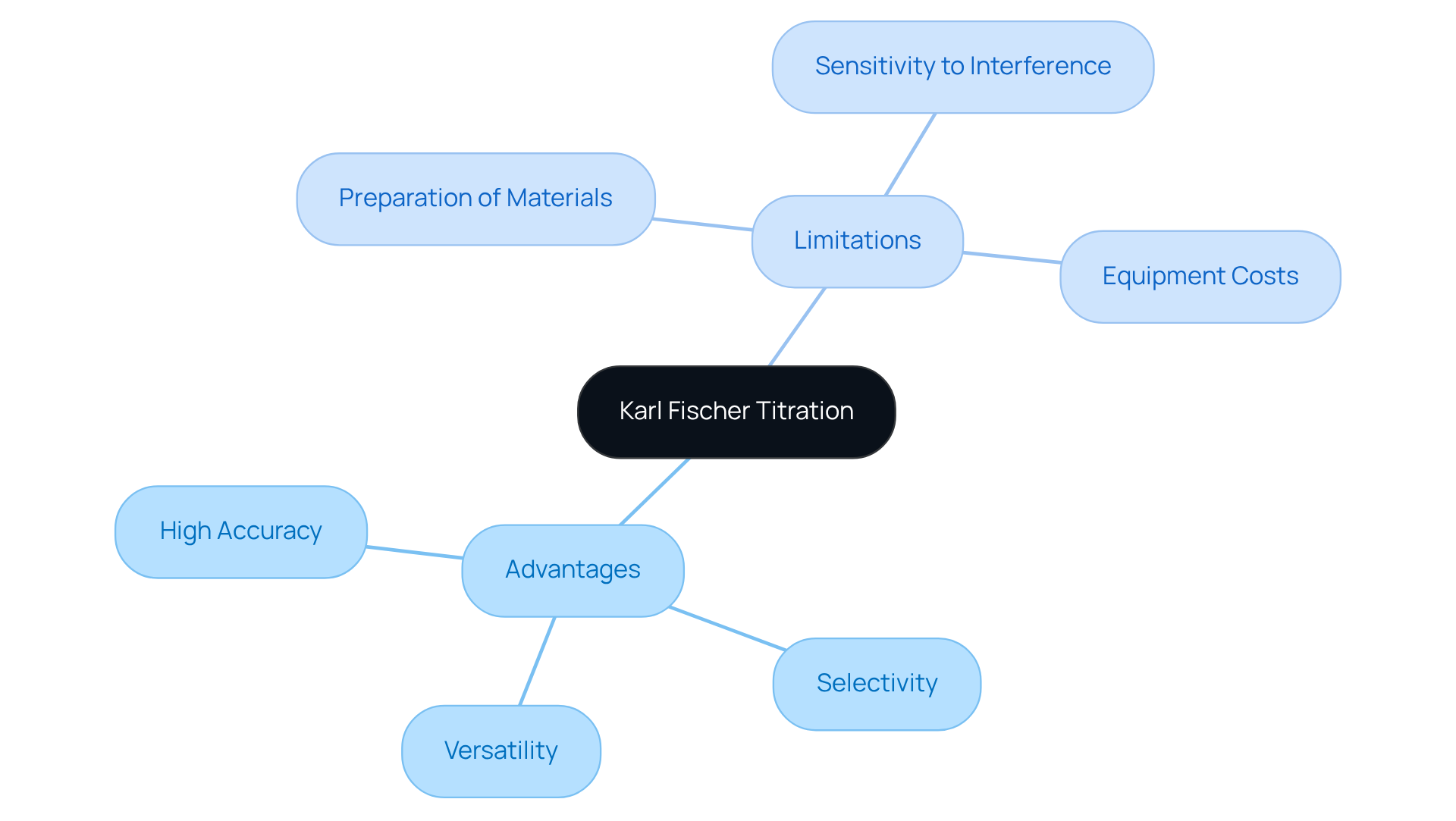
The karl fischer titration principle presents a range of advantages and limitations that laboratory professionals must carefully consider.
Advantages:
- High Accuracy: This method delivers precise measurements of moisture content, which is crucial for quality control across pharmaceuticals and various industries.
- Selectivity: It specifically targets water content, effectively minimizing interference from other substances.
- Versatility: The technique is applicable to a wide array of materials, encompassing solids, liquids, and gases.
Limitations:
- Preparation of Materials: Some specimens may require extensive preparation to ensure accurate results, potentially leading to time-consuming processes.
- Sensitivity to Interference: The presence of specific substances can affect measurement precision, necessitating meticulous sample selection and handling.
- Equipment Costs: The acquisition of high-quality titrators can be expensive, presenting a financial challenge for certain laboratories.
By comprehensively understanding these advantages and limitations, laboratory professionals can make informed decisions regarding the application of the karl fischer titration principle in their analytical processes.
Conclusion
Mastering the Karl Fischer titration principle is crucial for achieving success in laboratory moisture analysis. This technique offers unparalleled accuracy in measuring water content and serves as a benchmark method across various industries, including pharmaceuticals and food production. By comprehending the underlying chemical reactions and implementing best practices, laboratory professionals can guarantee precise and reliable results.
The article has delved into key insights, exploring everything from the foundational principles of the Karl Fischer titration to the distinctions between volumetric and coulometric methods. The emphasis on meticulous sample preparation, equipment calibration, and adherence to environmental conditions enhances the reliability of moisture analysis. Furthermore, the advantages and limitations of this method have been outlined, providing a comprehensive view that aids informed decision-making for laboratory applications.
Ultimately, the Karl Fischer titration principle remains an essential tool for laboratories striving for excellence in moisture determination. As the demand for accuracy in moisture analysis escalates, embracing this technique and its best practices will not only streamline laboratory workflows but also contribute significantly to overall quality assurance in product development and testing. By adopting these strategies, laboratory professionals will be empowered to navigate the complexities of moisture analysis with confidence and precision.




