Overview
This article presents an authoritative, step-by-step guide to mastering the titration lab procedure, a critical method for determining the concentration of an unknown solution through precise measurements and reactions. It underscores the necessity of meticulous preparation, execution, and error correction, bolstered by detailed instructions and insights into common pitfalls. Such guidance is designed to enhance the reliability and accuracy of titration results, ensuring that readers grasp the significance of each phase in the process.
Introduction
Understanding the titration lab procedure is essential for anyone involved in analytical chemistry; it serves as a cornerstone for determining the concentration of unknown solutions. This meticulous process not only enhances measurement accuracy but also ensures compliance with industry standards, making it invaluable for laboratory professionals.
However, despite its significance, many practitioners encounter challenges that can compromise their results.
What are the common pitfalls in titration, and how can they be effectively addressed to improve precision and reliability in laboratory settings? By recognizing these issues, laboratory professionals can enhance their methodologies and achieve superior outcomes.
Understand the Basics of Titration
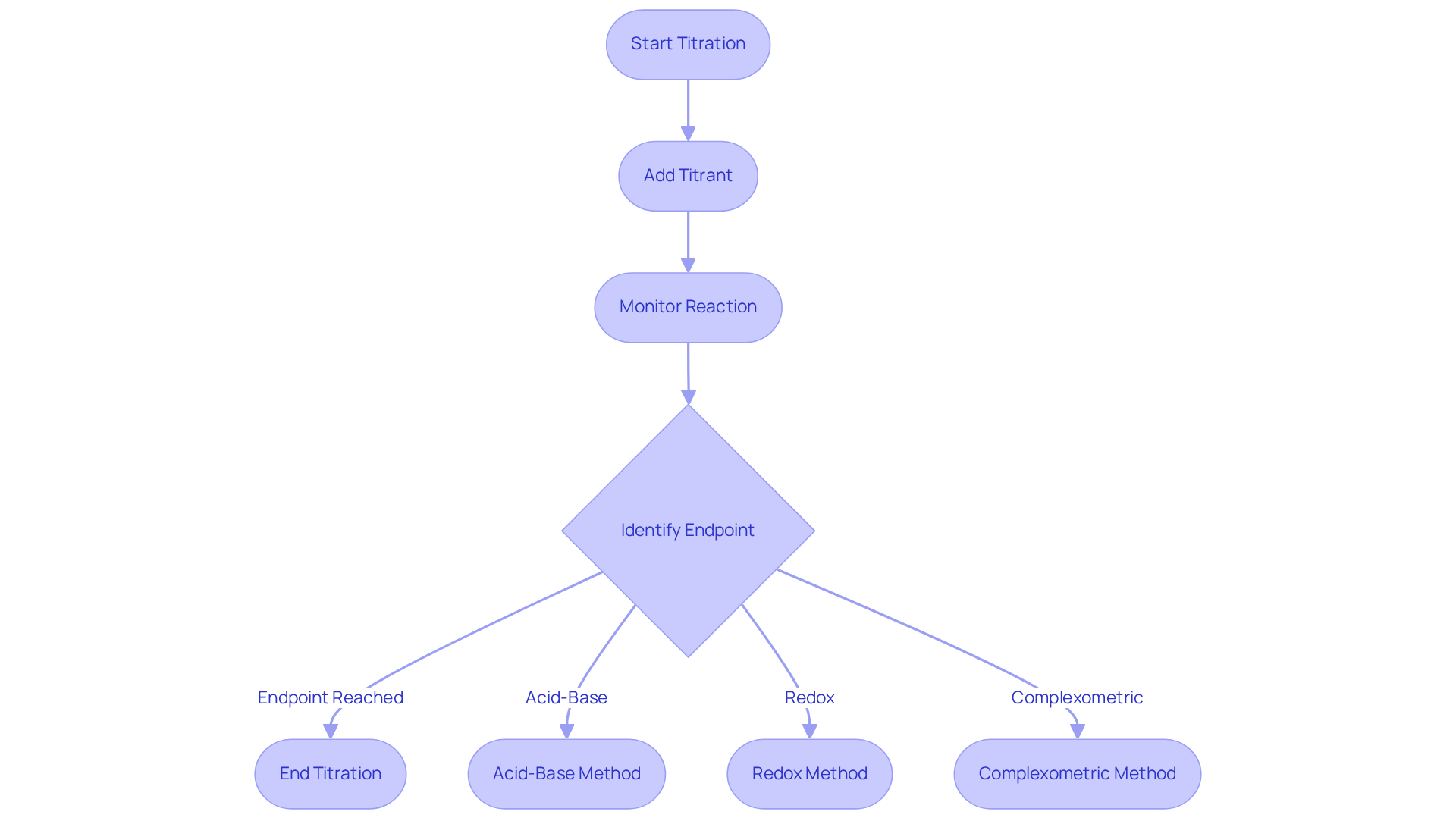
The titration lab procedure is a fundamental quantitative analytical technique used to determine the concentration of an unknown solution by reacting it with a solution of known concentration. This titration lab procedure necessitates the meticulous addition of the titrant to the analyte until the reaction achieves its endpoint, which is frequently indicated by a distinct color change. Various analytical methods exist, such as acid-base, redox, and complexometric analyses, each tailored for specific analytical objectives.
In 2025, it was estimated that approximately 75% of laboratories utilize volumetric analysis techniques, underscoring their critical role in analytical chemistry. For instance, Karl Fischer analysis serves as a classic method for determining trace amounts of water in samples, while redox analyses are vital for assessing substances like sulfur dioxide in wine, employing iodine and starch as indicators for accurate endpoint detection.
Understanding the titration lab procedure is crucial for laboratory professionals, as it enhances measurement precision and ensures compliance with industry standards. As one chemist aptly stated, 'The titration lab procedure is one of the oldest and most commonly used quantitative analytical methods,' which underscores its lasting relevance in laboratory practices. By mastering these techniques, laboratory managers can substantially enhance the reliability and efficiency of their analytical processes.
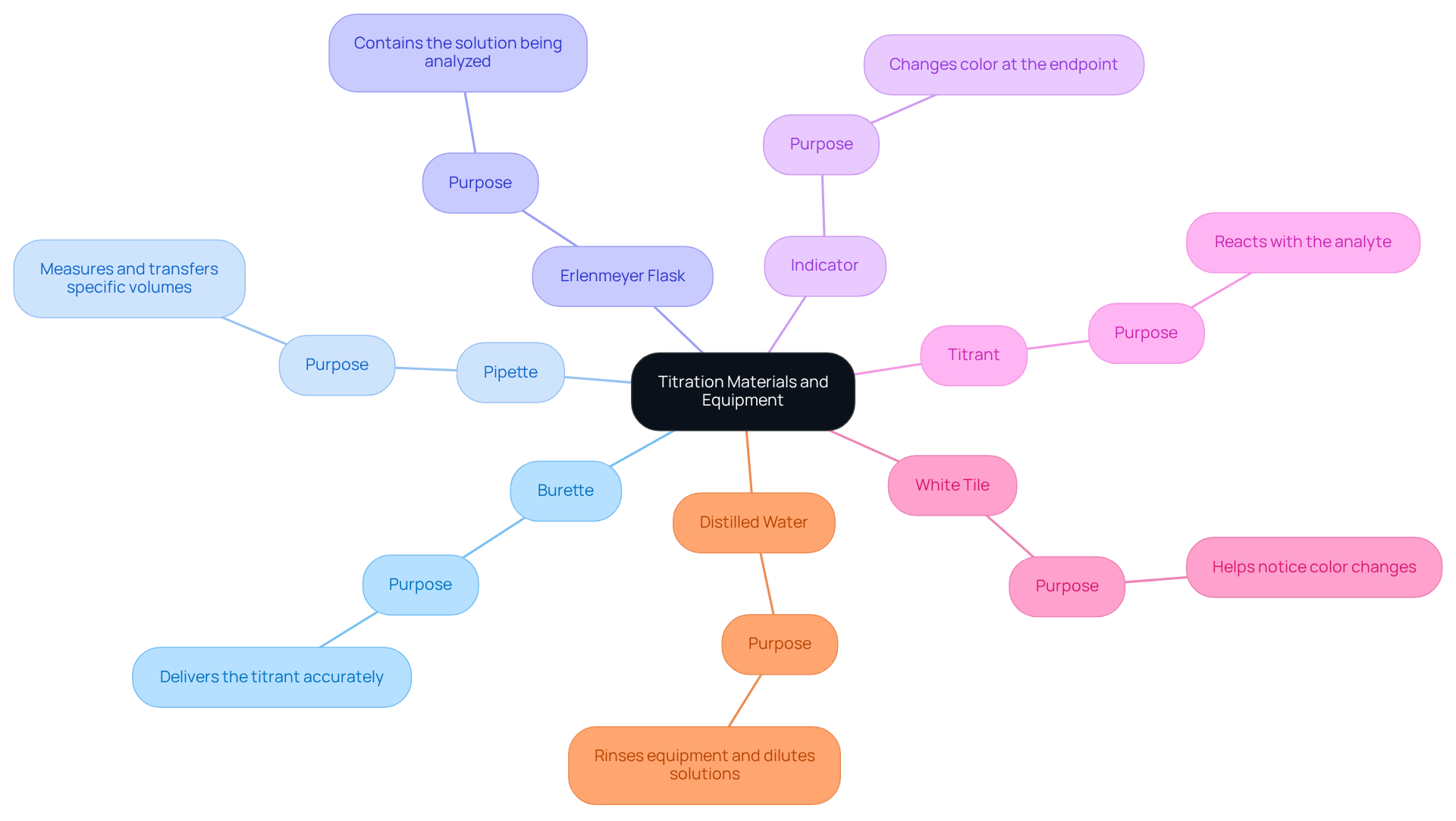
Gather Required Materials and Equipment
To perform a titration, it is essential to gather the following materials and equipment:
- A burette, which is a graduated glass tube with a tap at one end, used to deliver the titrant.
- A pipette, for accurately measuring and transferring a specific volume of the substance.
- An Erlenmeyer flask, designed to contain the solution being analyzed during the process.
- An indicator, such as phenolphthalein for acid-base analyses, which is crucial as it alters color at the endpoint.
- The titrant, a solution of known concentration, which will react with the analyte.
- A white tile, which aids in noticing color changes throughout the process.
- Distilled water, necessary for rinsing equipment and diluting solutions as needed.
Prior to commencing the titration, ensure that all equipment is clean and calibrated. This practice is vital to avoid contamination and inaccuracies, thereby upholding the integrity of the experiment.
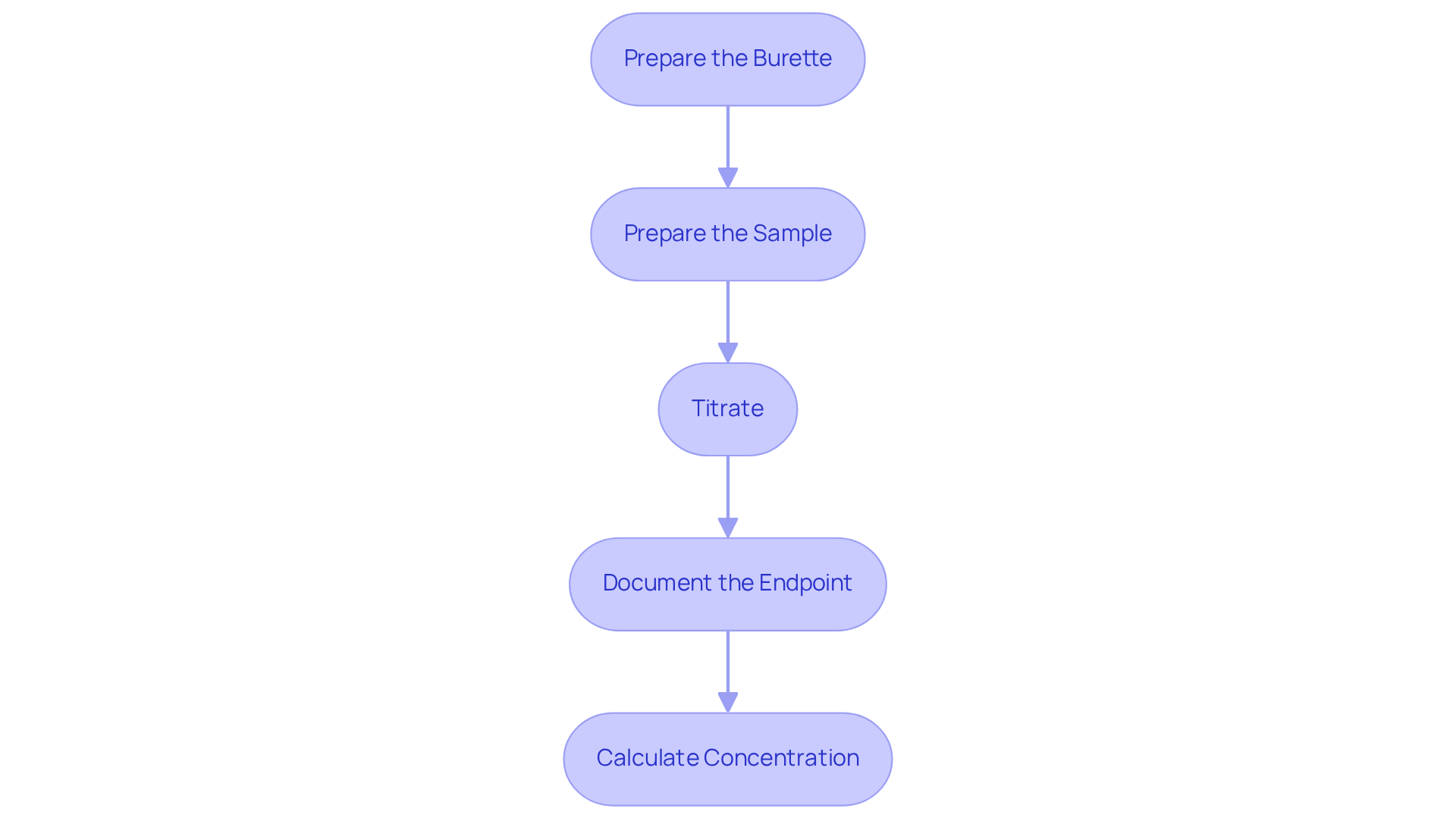
Follow the Step-by-Step Titration Procedure
To perform a titration effectively, follow these essential steps:
-
Prepare the Burette: Begin by rinsing the burette with distilled water, followed by rinsing it with the titration solution to prevent contamination. Fill the burette with the solution and accurately record the initial volume.
-
Prepare the Sample: Utilize a pipette to measure a precise volume of the sample solution into the Erlenmeyer flask. Add a few drops of the selected indicator to facilitate observation of the reaction.
-
Titrate: Gradually add the reagent from the burette to the analyte while continuously swirling the flask. Pay close attention to any color change, as this indicates the approach of the endpoint.
-
Document the Endpoint: Once the endpoint is reached—evidenced by a stable color change—document the final volume of the solution remaining in the burette.
-
Calculate Concentration: Finally, use the volume of titrant added along with its concentration to determine the concentration of the analyte with the formula:
C_1V_1 = C_2V_2In this equation,
C_1andV_1represent the concentration and volume of the titrant, whileC_2andV_2denote the concentration and volume of the analyte. This calculation is crucial for understanding the properties of the substances involved in the titration lab procedure.
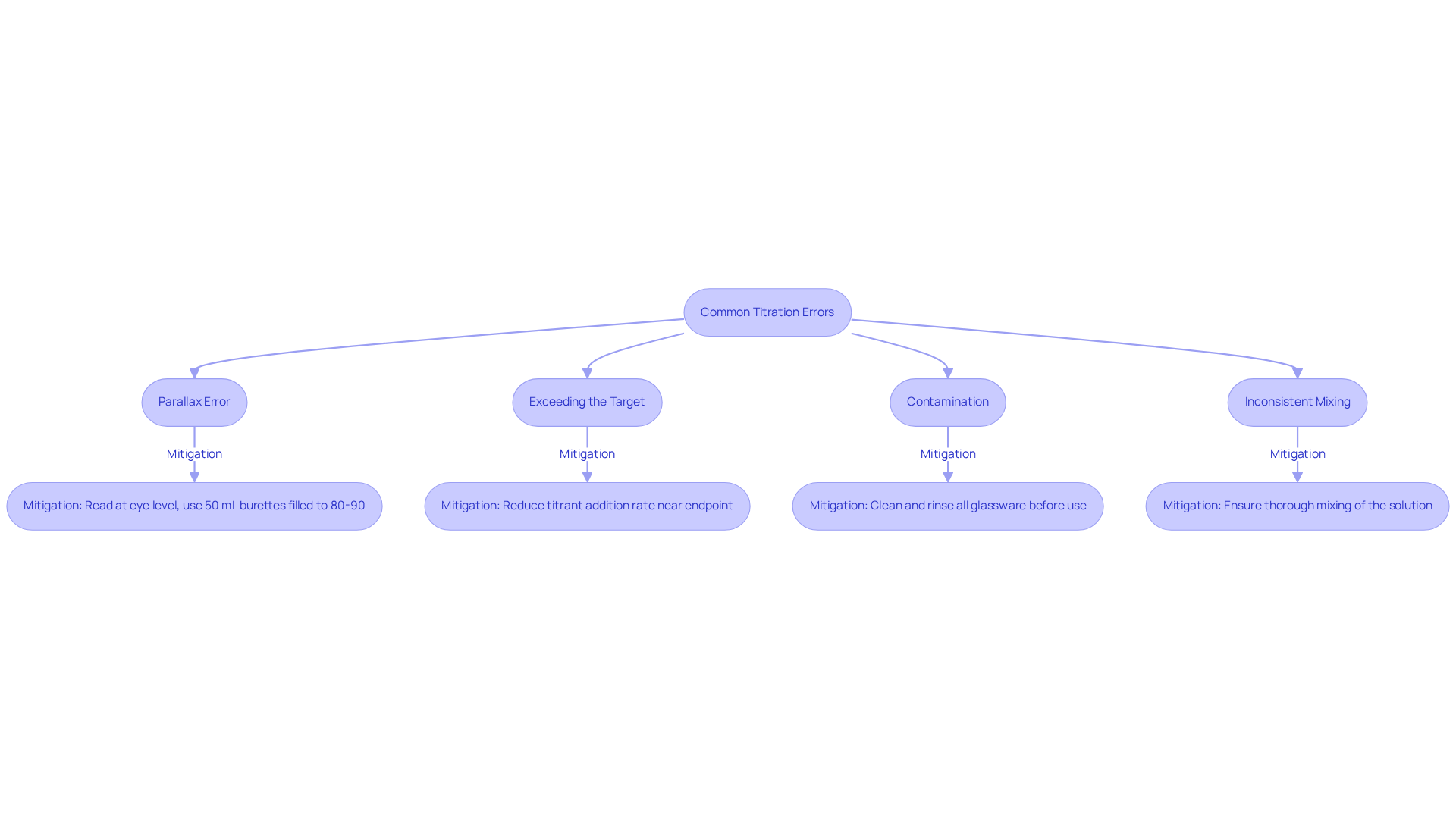
Identify and Correct Common Titration Errors
Frequent mistakes in the process can significantly impact the precision of outcomes. Key issues include the following:
- Parallax Error: This error arises when the burette is read from an angle, resulting in a misinterpretation of the liquid level. To mitigate this, it is essential to ensure that readings are taken at eye level. Additionally, utilizing 50 mL burettes filled to 80-90% of their volume can help minimize relative errors in titration.
- Exceeding the Target: Adding excess reagent beyond the target can distort results. To avoid this, reduce the titrant addition rate as you approach the endpoint, allowing for more accurate control. Misjudging the color of the indicator near the endpoint is a common human error that can lead to inaccuracies.
- Contamination: Impurities introduced by unclean equipment can compromise results. It is crucial to thoroughly clean and rinse all glassware before use.
- Inconsistent Mixing: Insufficient swirling of the flask can lead to uneven reactions, impacting the outcome of the process. Always ensure thorough mixing of the solution during the measurement process.
To effectively address these errors, practitioners should focus on refining their techniques. Utilizing large single volume pipettes (20 or 25 mL) can lead to smaller relative errors. Conducting initial tests can provide valuable insights into estimating the endpoint, thereby enhancing the precision of the actual measurement. Training sessions emphasizing these techniques can further reduce errors, fostering a culture of precision in laboratory practices. Moreover, case studies have demonstrated that intrinsic errors in titration methods, such as the endpoint not aligning with the equivalence point, can be mitigated through careful technique and training.
Conclusion
Mastering the titration lab procedure is essential for anyone engaged in analytical chemistry. This technique not only facilitates the precise determination of the concentration of unknown solutions but also reinforces adherence to industry standards. By following the systematic steps outlined—from preparing the necessary materials to accurately calculating concentrations—laboratory professionals can significantly enhance the reliability of their results.
The article delves into the intricacies of titration, underscoring the importance of understanding the equipment, recognizing common errors, and implementing best practices to mitigate inaccuracies. Key points include:
- The significance of using clean and calibrated tools
- The careful observation of color changes
- The necessity of consistent mixing
These insights empower practitioners to refine their techniques and improve their analytical outcomes.
Ultimately, mastering the titration lab procedure transcends merely following steps; it involves cultivating a mindset of precision and accuracy in laboratory practices. By embracing the principles discussed, chemists can elevate the quality of their work and contribute to the advancement of analytical chemistry. Engaging in continuous learning and practice will ensure that the art of titration remains a cornerstone of effective laboratory analysis.




