Overview
Mastering titrant solutions is pivotal in pharmaceutical laboratories, as it underpins the accurate determination of analyte concentrations through titration processes. This article delineates the essential procedures, various types of titrant solutions, and their applications. By emphasizing meticulous adherence to these procedures, it ensures robust quality control and compliance with industry standards. Such diligence ultimately safeguards drug efficacy and safety, reinforcing the critical nature of these practices in the pharmaceutical field.
Introduction
Titrant solutions are fundamental to precise analysis in pharmaceutical laboratories, serving a crucial function in determining the concentration of unknown substances through titration. Mastering the complexities of these solutions allows laboratory professionals to significantly improve the quality control processes essential for ensuring the safety and efficacy of medications.
However, the challenge arises in navigating the various types of titrant solutions and their specific applications. This raises an important question: how can one effectively implement these solutions to uphold rigorous industry standards?
Define Titrant Solutions and Their Role in Titrations
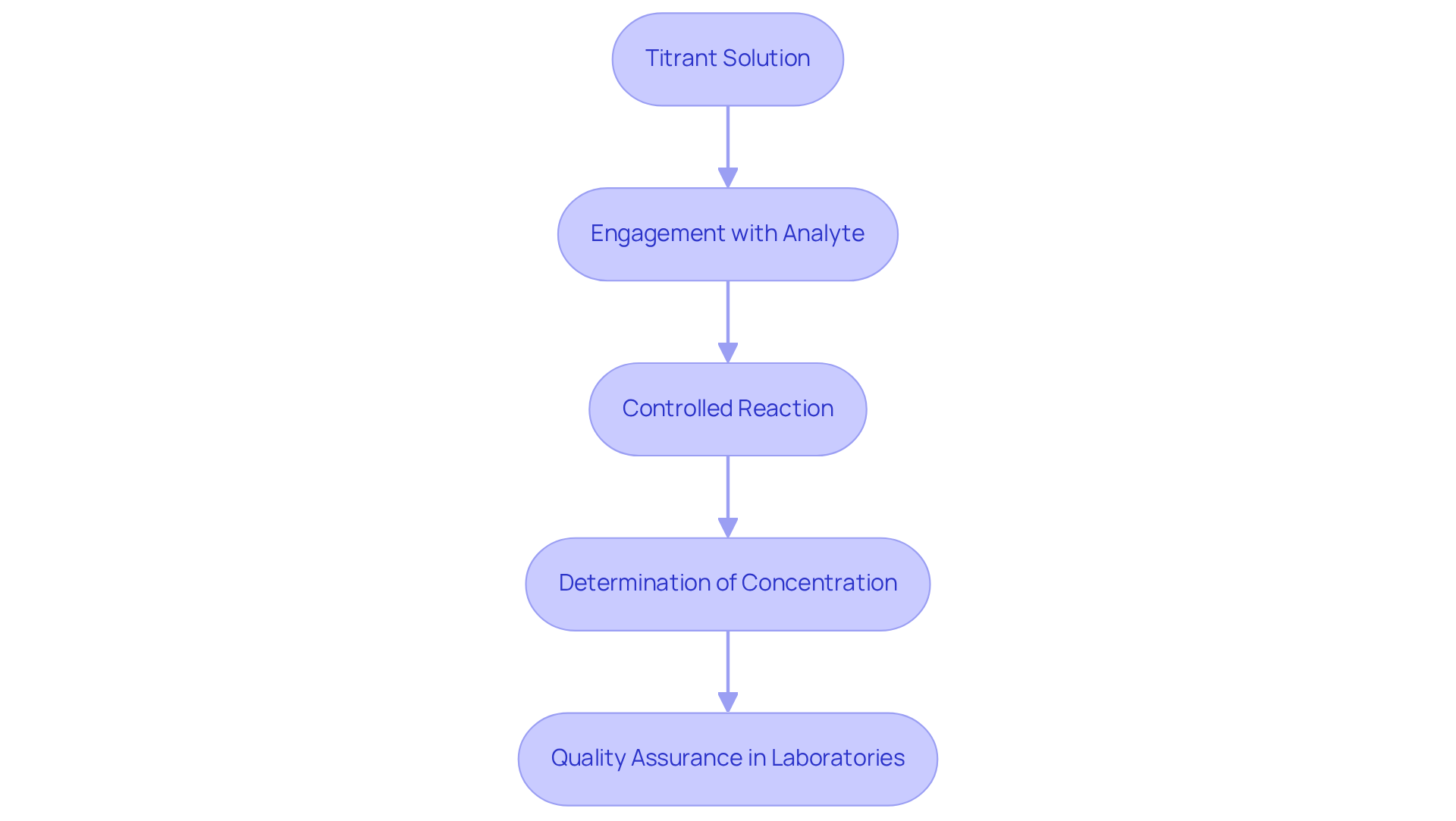
Titrant solutions act as calibrated solutions with a known strength, which play a critical role in titrations to accurately determine the amounts of unknown analytes. Their primary function is to engage with the titrant solution in a controlled manner, facilitating precise assessment of the analyte's concentration.
In drug laboratories, the use of a titrant solution is indispensable for quality assurance, ensuring that the active components in medications meet established levels. For instance, in acid-base titrations, a strong acid or base serves as the titrant solution, neutralizing the analyte and enabling an accurate determination of concentration based on the quantity of titrant solution used at the reaction's endpoint.
This meticulous process is vital for preserving the integrity of pharmaceutical products, as it directly influences their efficacy and safety. Furthermore, the application of Karl Fischer techniques, particularly with the Hiranuma Aquacounter AQV-300 Volumetric and AQ-300 Coulometric analyzers, is crucial for evaluating moisture levels in pharmaceuticals, thereby ensuring compliance with the Japanese Pharmacopoeia.
Statistics reveal that adherence to stringent dosing protocols can significantly bolster the reliability of quality control measures, thereby supporting compliance with industry standards and regulations.
Explore Different Types of Titrant Solutions and Their Applications
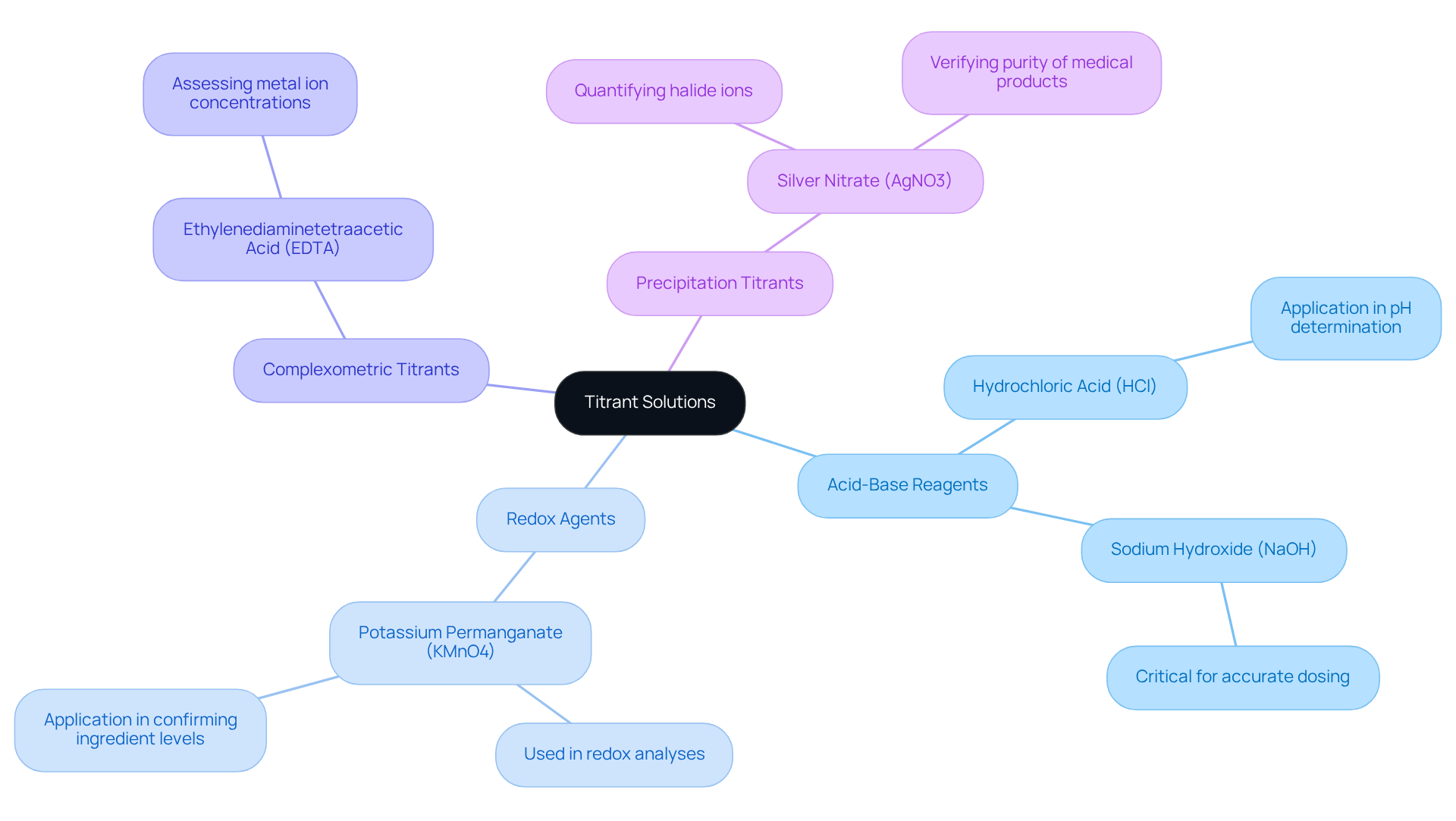
Titrant solutions are categorized according to their chemical characteristics and the specific reactions they enable, with each playing a vital role in drug analysis. Understanding these classifications is essential for laboratory experts aiming to enhance quality control processes in pharmaceuticals.
Acid-Base Reagents are extensively used in acid-base analyses. Reagents like hydrochloric acid (HCl) and sodium hydroxide (NaOH) are vital for determining the pH of solutions. Their application in drug quality control is indispensable, as accurate dosing methods are critical in an industry where the margin for error is exceptionally narrow. Industry leaders emphasize the importance of precision in drug production to ensure product consistency and efficacy.
Redox Agents, such as potassium permanganate (KMnO4), are employed in redox analyses to ascertain the oxidation state of analytes. This analysis is particularly significant for substances undergoing oxidation-reduction reactions, which are prevalent in pharmaceutical formulations. A case study titled 'Quality Control in Pharmaceuticals Using Titration' illustrates the practical application of redox titrations to confirm the correct levels of ingredients in drug formulations.
Complexometric Titrants like ethylenediaminetetraacetic acid (EDTA) serve a pivotal role in assessing metal ion concentrations in solutions. This is especially important in medical applications where controlling metal ion content is essential. The necessity for quality assurance in medical products underscores the significance of regulating metal ion levels.
Precipitation Titrants, such as silver nitrate (AgNO3), are frequently utilized in precipitation titrations to quantify halide ions in solutions. This method is crucial for verifying the purity of medical products, as it aids in identifying impurities that could compromise drug safety and effectiveness. The relevance of precipitation titrants is highlighted by Jessica Clifton, who notes that both patients and regulatory bodies depend on the integrity of every medicine produced.
By comprehending these various types of titrant solutions and their applications, laboratory experts can make informed decisions when selecting the appropriate titrant for specific analytical needs. This knowledge ultimately enhances quality control procedures in drug laboratories. Current trends indicate an increasing reliance on automated measuring techniques, which significantly improve precision and efficiency in pharmaceutical analysis.
Implement Titration Procedures: Step-by-Step Guide
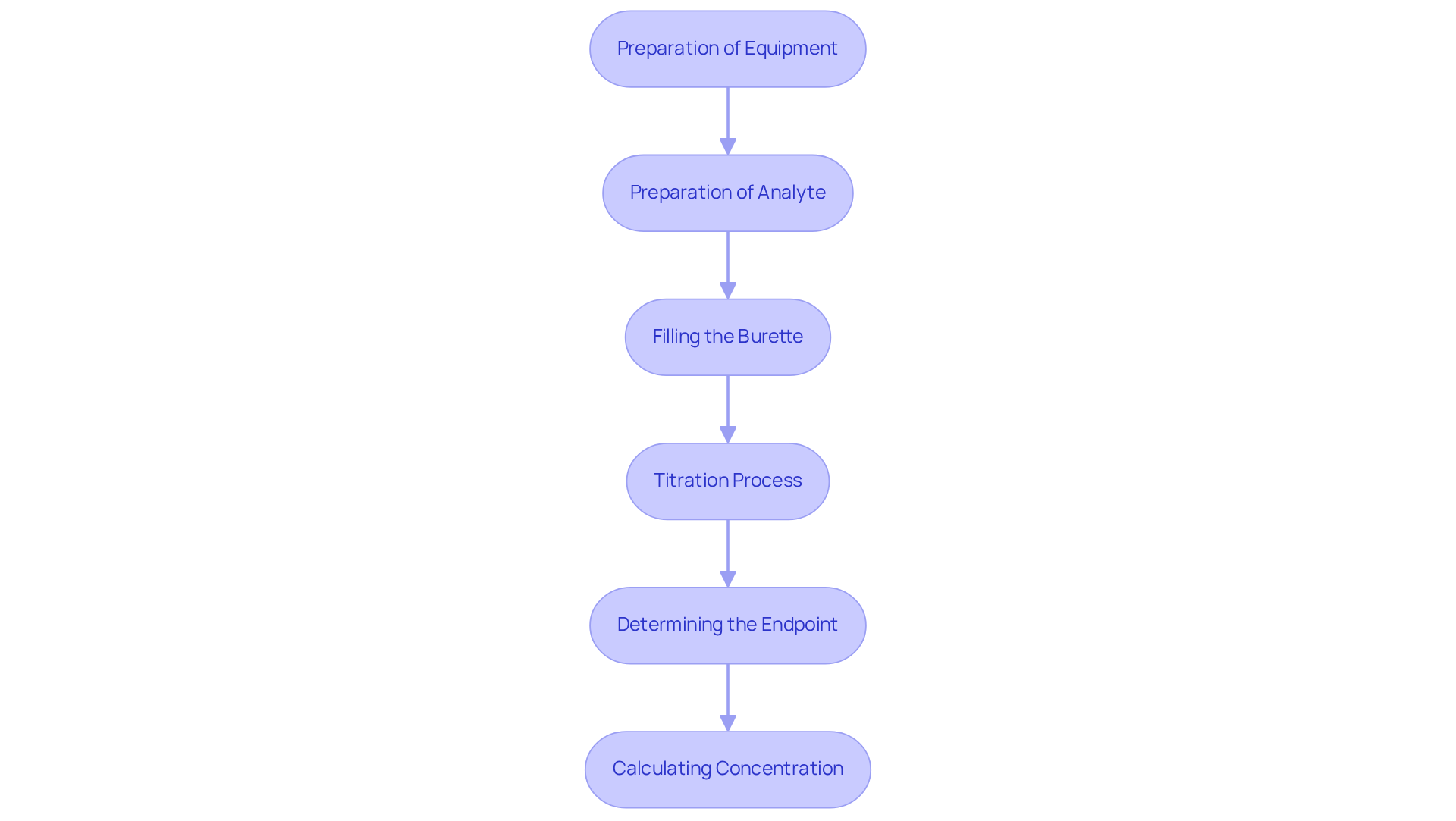
To implement titration procedures effectively, it is essential to adhere to the following steps:
-
Preparation of Equipment: Begin by ensuring that all glassware, including burettes, pipettes, and flasks, is meticulously cleaned and free from contaminants. Rinse the burette with the titrant solution to prevent dilution and ensure accuracy. Utilizing premium titrators from JM Science Inc. can significantly enhance the reliability of your results.
-
Preparation of Analyte: Accurately measure a specific volume of the analyte solution using a pipette and transfer it to an Erlenmeyer flask. Incorporate a few drops of an appropriate indicator, such as bromothymol blue or phenolphthalein, which will signal the endpoint of the titration.
-
Filling the burette with the titrant solution requires careful attention to ensure that no air bubbles are present in the nozzle. Record the initial volume of the solution in the burette for precise calculations.
-
In the titration process, gradually add the titrant solution to the analyte solution while continuously swirling the flask to ensure thorough mixing. As you approach the endpoint, add the reagent dropwise, observing for a color change in the solution.
-
Determining the Endpoint: Cease adding the reagent once the endpoint is reached, indicated by a stable color change. Document the final volume of the solution in the burette to facilitate precise calculations.
-
Calculating Concentration: Utilize the volume of titrant added and its concentration to determine the concentration of the analyte using the formula:
C1V1 = C2V2Here, C1 and V1 represent the concentration and volume of the titrant, while C2 and V2 denote the concentration and volume of the analyte.
By adhering to these procedures, laboratories can guarantee precise and repeatable outcomes in measurement experiments, which are crucial for upholding high standards in the quality control of medications. Moreover, employing automation solutions like the OMNIS Sample Robot enhances efficiency, allowing for the analysis of up to 175 samples simultaneously, thereby saving up to 60% of the time compared to traditional methods. This approach not only streamlines processes but also aligns with the rigorous standards set by pharmacopoeias for drug quality and safety. Additionally, using high-quality reagents, such as distilled water with a typical TDS value of less than 0.5 ppm, is critical for maintaining accuracy in titration procedures. Furthermore, strict adherence to the rules for measuring the conductivity of water for pharmaceutical use, as outlined in USP<645>, ensures compliance with regulatory standards.
Conclusion
Titrant solutions are indispensable tools in pharmaceutical laboratories, acting as calibrated agents that enable the precise determination of unknown analyte concentrations. Their critical role in quality assurance is paramount, ensuring that medications adhere to established efficacy and safety standards. By employing various types of titrant solutions—ranging from acid-base reagents to complexometric and precipitation titrants—laboratories can conduct accurate analyses that uphold the integrity of pharmaceutical products.
This article has explored the essential functions of titrant solutions, emphasizing their applications across various titration methods vital for drug analysis. The importance of adhering to meticulous procedures and utilizing high-quality reagents has been underscored, as these practices directly influence the reliability of quality control measures. Furthermore, advancements such as automated titration systems have been discussed, illustrating how they enhance both efficiency and accuracy in pharmaceutical testing.
In conclusion, mastering titrant solutions is crucial for pharmaceutical labs dedicated to maintaining high standards in drug quality and safety. By comprehending the nuances of different titrant types and adhering to best practices in titration procedures, laboratory professionals can significantly enhance their analytical capabilities. This commitment to precision and quality not only benefits the pharmaceutical industry but also safeguards public health, highlighting the essential nature of rigorous quality control in medication production.




