Overview
The article centers on mastering titration methods to achieve accurate and compliant results in analytical chemistry. It underscores the significance of employing proper techniques and adhering to regulatory standards, alongside the integration of advanced instruments. Collectively, these elements enhance precision and reliability in titration processes. Best practices and case studies highlighted throughout the content further support these assertions, demonstrating the critical role that high-quality scientific instruments play in laboratory settings.
Introduction
In the realm of analytical chemistry, titration stands as a cornerstone technique, pivotal for determining the concentration of unknown solutions with remarkable precision. This method involves the careful addition of a titrant to a solution until a specific endpoint is reached. It is not only fundamental but also multifaceted, encompassing various types such as:
- acid-base
- redox
- complexometric titrations
As laboratories strive for greater efficiency and accuracy, the integration of advanced technologies and adherence to regulatory standards have become essential. This article delves into the intricacies of titration, exploring its foundational principles, strategies for achieving accurate results, compliance with regulatory requirements, and the transformative role of modern instruments in enhancing laboratory practices.
Through a comprehensive examination of these elements, it becomes clear that mastering titration is critical for ensuring reliable analytical outcomes in diverse scientific applications.
Understand Titration Fundamentals and Techniques
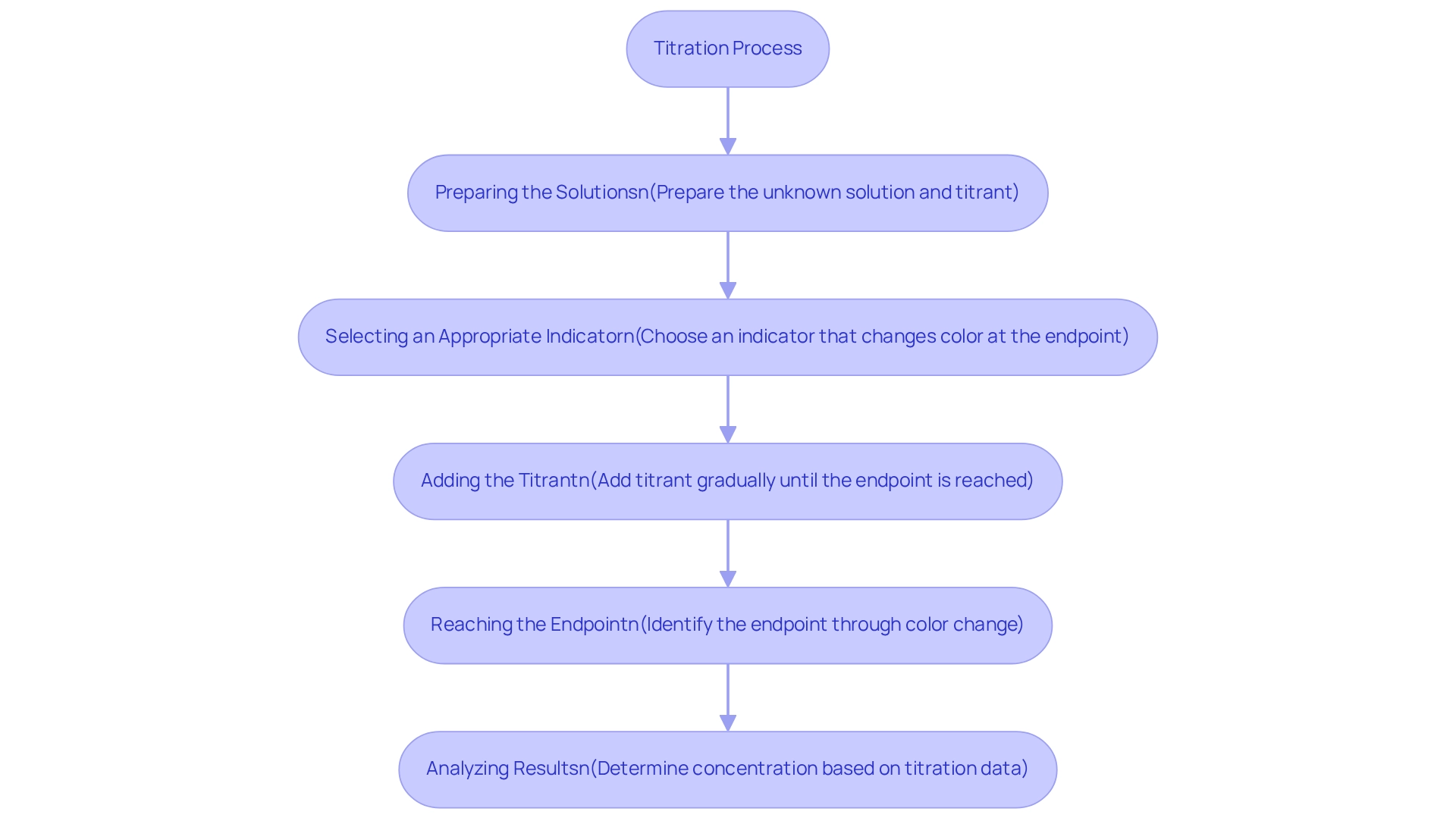
Titration methods serve as , essential for determining the concentration of an unknown solution through its reaction with a titrant of known concentration. This process involves several critical steps:
- Preparing the solutions
- Selecting an appropriate indicator
- Meticulously adding the titrant until the endpoint is reached, typically indicated by a color change
Various types of analysis, including titration methods such as acid-base, redox, and complexometric methods, are designed for specific applications, underscoring the versatility of these titration methods in analytical chemistry.
Understanding these essentials is vital for achieving measurements with accuracy and effectiveness. Key concepts such as molarity, defined as the number of moles of solute per liter of solution, and normality, which denotes the concentration equivalent of a solution, significantly influence the choice of analytical methods. Recent statistics reveal that a substantial percentage of laboratories are now employing automated measurement techniques, including the AQ-300 Coulometric and AQV-300 Volumetric Karl Fischer devices from JM Science. This trend reflects an ongoing shift towards enhanced efficiency and accuracy in analytical processes, particularly in drug and medicine testing, in compliance with the Japanese Pharmacopoeia.
Real-world applications of volumetric analysis techniques can be illustrated through case studies, such as the limit test for sulfate. This test involves the reaction between barium chloride and soluble sulfates to produce barium sulfate, where the turbidity of the test solution is compared to a standard sulfate solution to determine compliance with established limits. Such examples highlight the significance of precise execution in attaining dependable analytical findings, as careful measurement is crucial for accurate outcomes.
As the field of analytical chemistry evolves, advancements in measurement methods continue to emerge, enhancing laboratory capabilities. Industry leaders advocate for best practices that incorporate cutting-edge technologies, such as those provided by JM Science, ensuring that processes remain fundamental for precise and compliant analytical outcomes. Insights from renowned chemists emphasize that "innovation is the key to unlocking new realms of chemistry; as we embrace technology, we redefine what's possible." This perspective reinforces the continuous commitment to excellence in measurement methodologies.
Implement Strategies for Accurate Titration Results
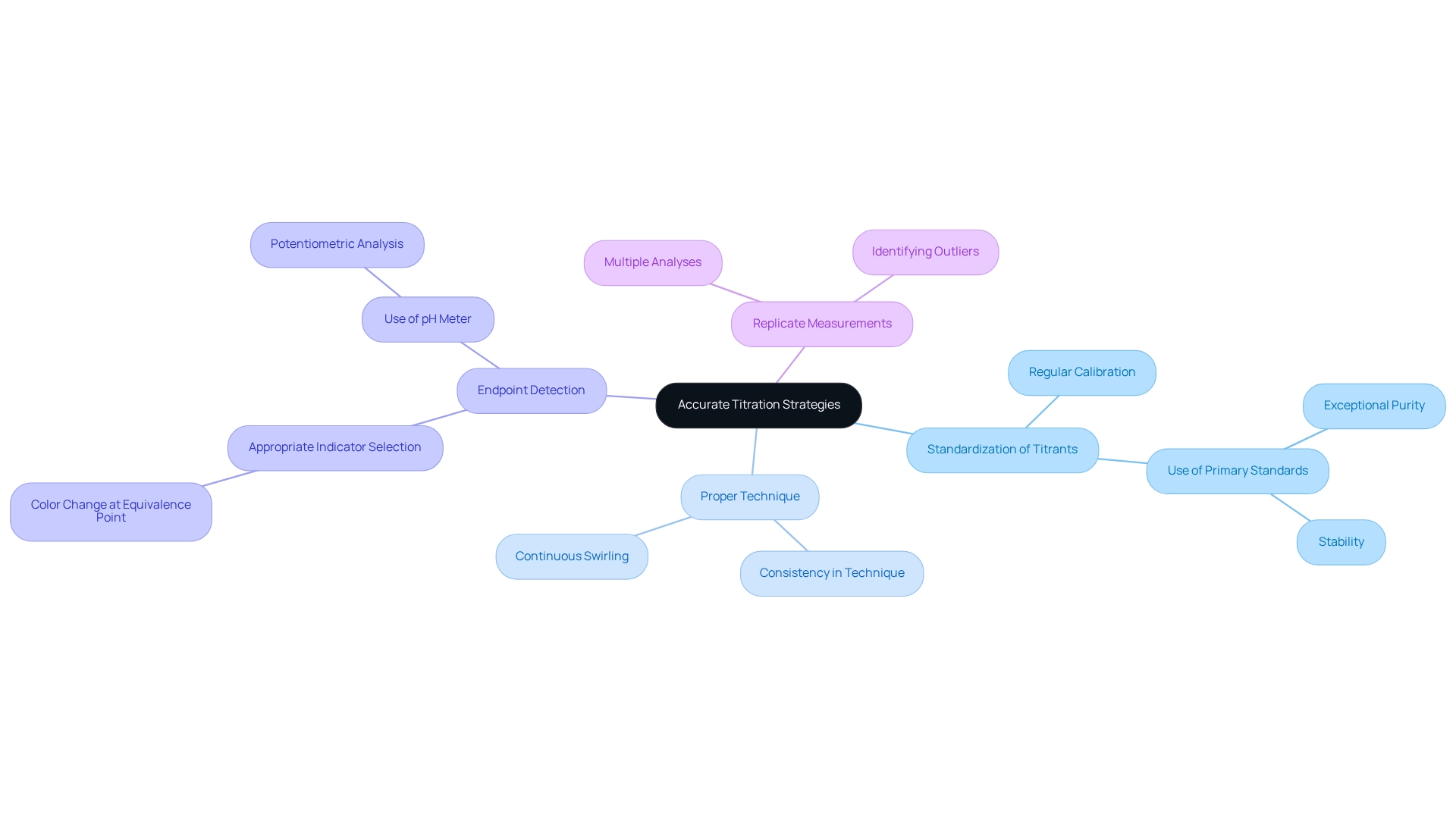
Achieving requires the use of various titration methods that are essential for laboratory precision.
- Standardization of Titrants is paramount. Regular calibration and standardization of titrant solutions ensure accurate concentrations. Utilizing primary standards that are exceptionally pure and stable is a best practice that significantly enhances the reliability of measurement results.
- Next, Proper Technique plays a crucial role. Consistency in technique when adding titrant cannot be overstated. Continuously swirling the flask during the titration promotes thorough mixing of solutions, facilitating a uniform reaction and thereby improving accuracy.
- Furthermore, Endpoint Detection is vital for successful analysis. Selecting the appropriate indicator for your specific type of analysis is critical. The chosen indicator must exhibit a color change at a pH close to that of the equivalence point. Familiarity with the color change associated with the endpoint is essential for achieving accurate results. For enhanced accuracy, employing a pH meter in potentiometric analysis can provide more reliable endpoint detection.
- Additionally, Replicate Measurements are a recommended practice. Conducting multiple analyses on the same sample ensures consistency and aids in identifying outliers, thereby strengthening the trustworthiness of the findings.
Integrating these strategies not only aligns with best practices but also meets the essential requirement for standardization in titration methods. This is underscored by case studies demonstrating that proactive actions can significantly reduce errors related to reagents and equipment. Regular titer determination is particularly crucial for sensitive titrants like iodine or DPIP, which are susceptible to UV radiation and oxygen exposure. By focusing on these techniques, laboratories can markedly enhance the precision and reliability of their measurements. As Benjamin Franklin wisely stated, "An investment in knowledge pays the best interest," highlighting the importance of understanding and implementing these strategies.
Ensure Compliance with Regulatory Standards in Titration
Adherence to regulatory standards in measurement is paramount for ensuring the precision and reliability of outcomes. Laboratories must comply with guidelines established by organizations such as the FDA and ISO. Key practices include:
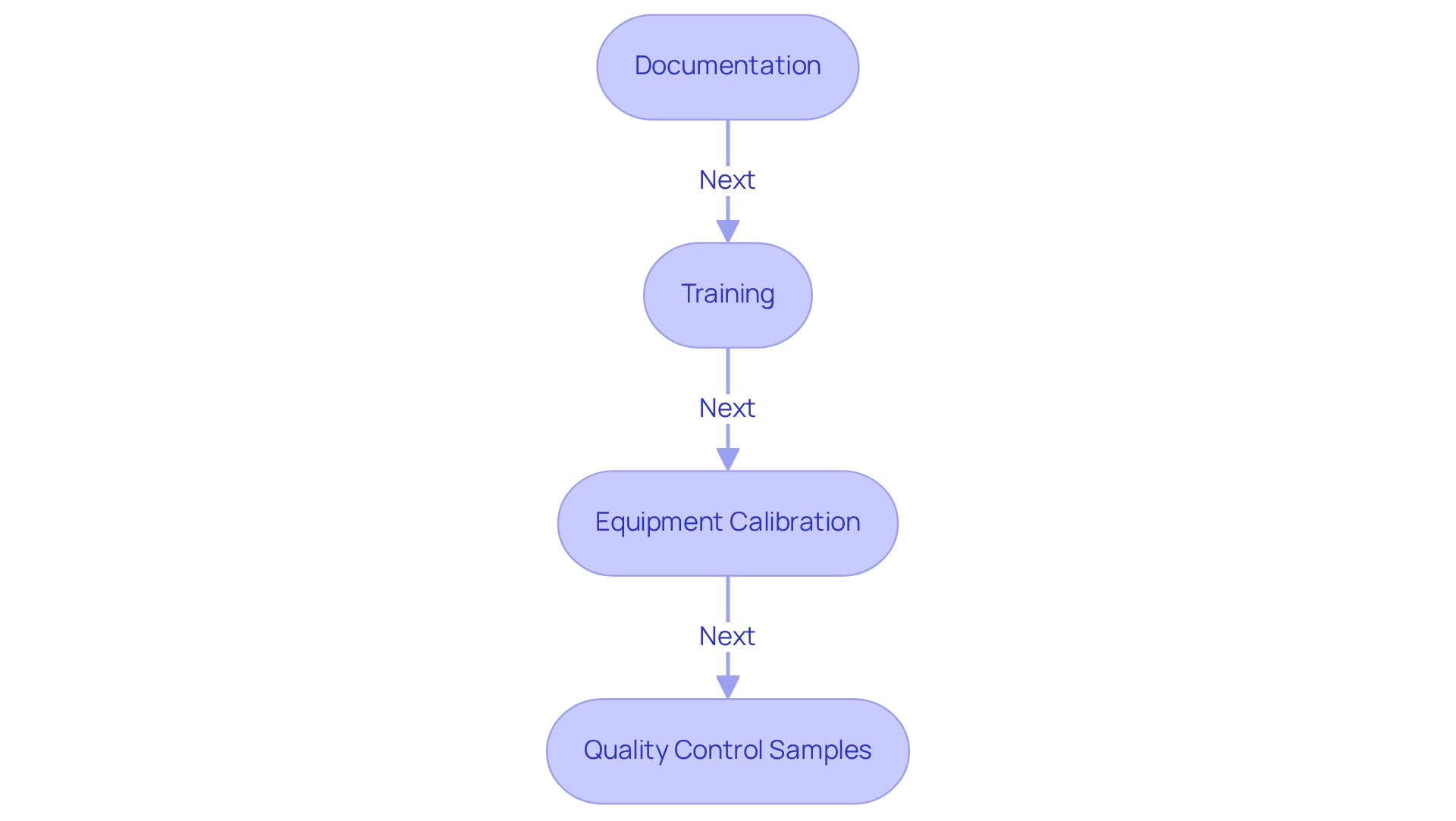
- Documentation of all titration methods is essential for maintaining comprehensive records of the procedures. This includes documenting reagent lot numbers, calibration data, and results. Such thorough documentation is crucial for audits and traceability, with studies indicating that over 80% of laboratories prioritize this practice to meet compliance standards. Furthermore, utilizing a white tile beneath the flask can enhance the visibility of color changes during experiments, aiding in more precise evaluations.
- Training: It is imperative that all personnel involved in titration methods are adequately trained in both the techniques and the regulatory requirements. Frequent training sessions not only enhance skill levels but also ensure that personnel remain current with the latest compliance protocols, fostering a culture of safety and precision in work environments. titled 'Integration of Safety in Laboratory Practices' underscores the significance of safety protocols when managing chemicals, promoting a culture of safety in testing environments.
- Equipment Calibration: Routine adjustment of all devices utilized in measuring, including burettes, pipettes, and pH meters, is essential to meet precision standards. Following FDA guidelines, facilities should establish a calibration schedule that aligns with best practices to ensure consistent performance.
- Quality Control Samples: Employing quality control samples is an essential practice for confirming the precision of measurement outcomes. This approach aids in identifying any deviations from expected results, reinforcing the reliability of the data produced. The incorporation of quality control measures has been shown to significantly enhance the credibility of test outcomes, as emphasized in recent case studies on adherence to FDA and ISO standards.
As chemist Robert H. Grubbs observed, 'The more trials, the better the average,' highlighting the importance of detailed documentation and repeated experiments in measurement processes. By implementing these titration methods, laboratories can ensure adherence to regulatory standards while also elevating the overall quality of their analytical processes, ultimately contributing to advancements in research and healthcare. Moreover, the significance of acid-base experiments in advancing scientific understanding underscores the vital role that compliance plays in the broader scientific framework.
Leverage Advanced Instruments for Enhanced Titration Practices
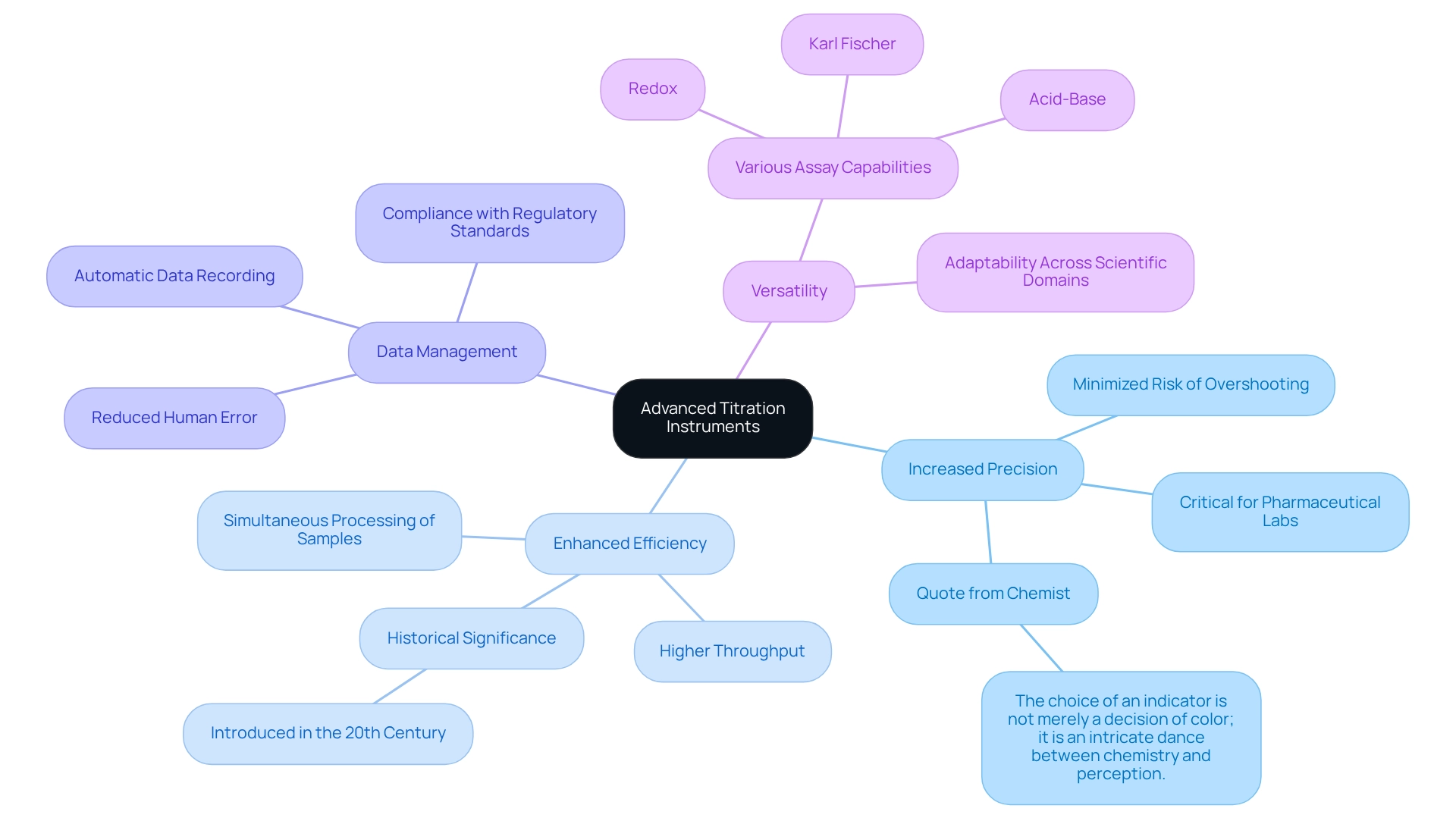
Advanced titration instruments, including automated titrators and digital burettes from JM Science Inc., offer substantial advantages over traditional methods. These instruments can significantly enhance laboratory practices in several ways.
- Increase Precision: Automated titrators provide exceptional control over titrant addition, thereby minimizing the risk of overshooting the endpoint. This precision is critical in pharmaceutical laboratories where accuracy is non-negotiable. As a renowned chemist aptly stated, "The choice of an indicator is not merely a decision of color; it is an intricate dance between chemistry and perception," underscoring the complexities involved in achieving reliable results.
- Enhance Efficiency: The automation of measurement processes enables higher throughput, allowing for the simultaneous processing of multiple samples. This capability is particularly beneficial in high-volume settings, where effective time and resource management are essential. The introduction of automated titrators in the 20th century marked a pivotal advancement in laboratory efficiency and precision.
- Data Management: Modern titrators, including those from JM Science, come equipped with sophisticated software that automatically records data, thereby reducing human error and streamlining data analysis. This feature not only bolsters accuracy but also aids in maintaining compliance with regulatory standards.
- Versatility: are capable of executing a variety of assays, including acid-base, redox, and Karl Fischer methods. This adaptability makes them suitable for diverse applications across multiple scientific domains, effectively addressing the evolving needs of research facilities.
In addition to titration instruments, JM Science offers a comprehensive range of products, such as HPLC columns and accessories, further enhancing testing capabilities. The competitive pricing of these products provides substantial benefits for facilities aiming to improve their operations.
The evolution of automated titrators has revolutionized laboratory practices, with manufacturers like JM Science focusing on user-friendly designs and improved reagent handling systems. Insights from the case study titled "Technological Advancements in Measurement Devices" indicate that ongoing innovations in measurement technology, including digital interfaces and enhanced reagent handling systems, are significantly improving device functionality. Consequently, the market for laboratory titration devices is projected to experience considerable growth, driven by the expansion of research facilities and the increasing demand for precision in analytical processes. Feedback from laboratory managers emphasizes the efficiency gains realized through the use of automated titrators, highlighting their crucial role in boosting laboratory productivity and accuracy.
Conclusion
The exploration of titration techniques underscores their fundamental importance in analytical chemistry, highlighting the precision necessary for determining the concentration of unknown solutions. Mastery of essential principles—ranging from the standardization of titrants to proper technique and endpoint detection—is crucial for achieving reliable results. Moreover, the integration of advanced instruments has transformed traditional practices, significantly enhancing both accuracy and efficiency in laboratory environments.
Compliance with regulatory standards is equally vital, as it ensures the credibility and reliability of analytical outcomes. By adhering to best practices, including thorough documentation and regular training, laboratories can not only meet compliance requirements but also foster a culture of safety and excellence. The emphasis on quality control and equipment calibration further underscores the commitment to producing trustworthy results across diverse scientific applications.
Ultimately, the continuous evolution of titration methodologies, bolstered by technological advancements, positions titration as an indispensable tool in the pursuit of precision within analytical processes. As laboratories embrace innovative solutions and uphold rigorous standards, the importance of mastering titration techniques becomes increasingly apparent, paving the way for advancements in research and healthcare. By prioritizing accuracy and compliance, the analytical community can ensure reliable outcomes that contribute to the broader scientific landscape.




