Overview
This article delves into the setup, calibration, and maintenance of UV/Visible detectors, underscoring their vital role in analytical chemistry and medical diagnostics. It begins by introducing essential principles, notably the Beer-Lambert Law, which is crucial for accurate measurement interpretation. The discussion extends to various detector types and their specific applications, illustrating the breadth of their utility in scientific analysis. Furthermore, the article provides a systematic approach to calibration and troubleshooting, ensuring consistent reliability in analytical results. By understanding these elements, professionals can enhance the quality of their laboratory work and uphold the integrity of their findings.
Introduction
Understanding the intricacies of UV/visible detectors is paramount for anyone involved in analytical chemistry or medical diagnostics. These sophisticated instruments depend on the absorption of light to provide critical data; however, their effectiveness hinges on proper setup, calibration, and maintenance. This article delves into the essential principles of UV/visible detection, explores the various types of detectors available, and offers a comprehensive guide to ensuring optimal performance. As technology advances, users must navigate the complexities of calibration and troubleshooting to maintain accuracy and reliability in their results.
Explore the Principles of UV/Visible Detection
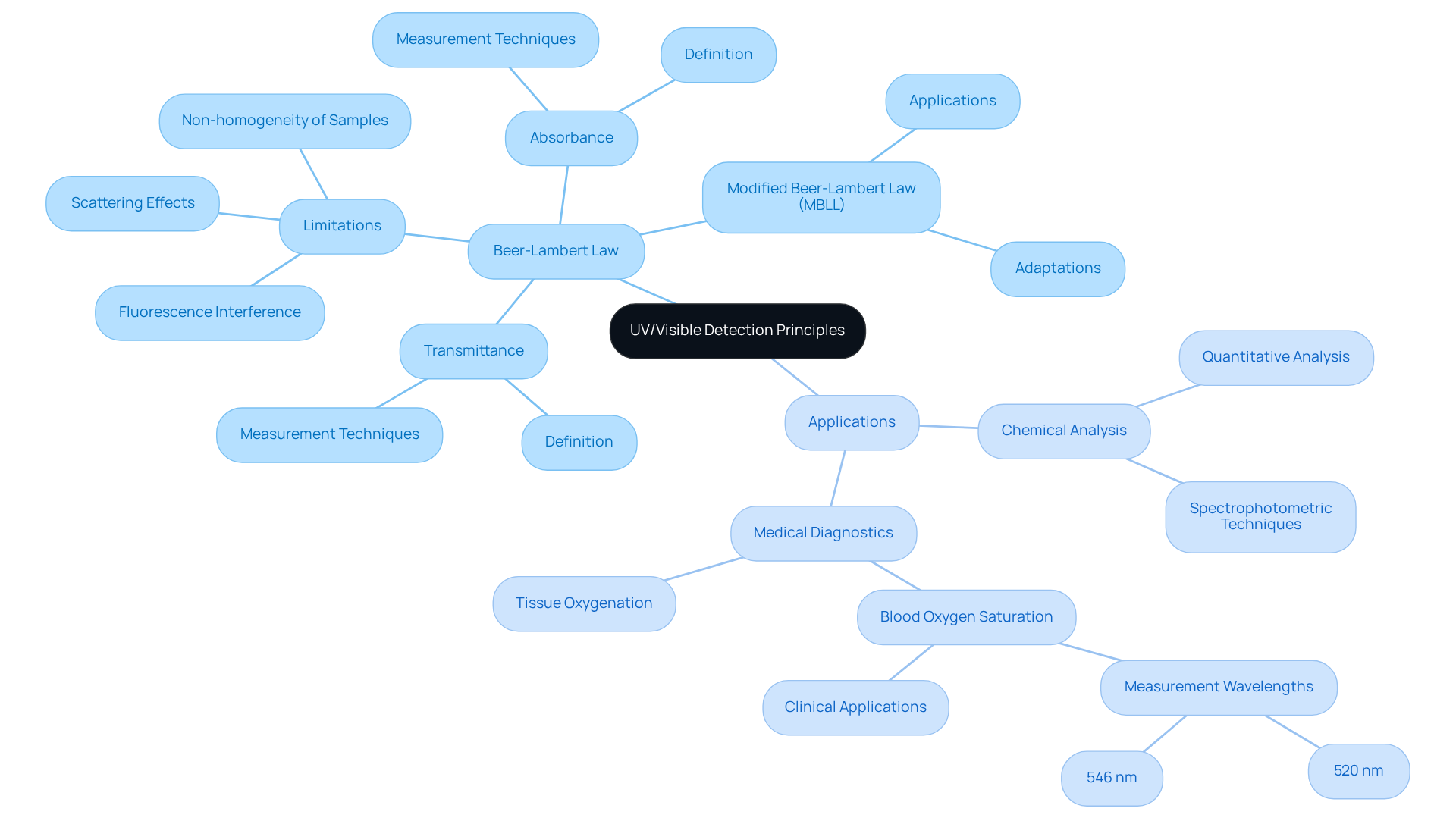
The absorption of radiation by molecules within the ultraviolet (UV) and visible regions of the electromagnetic spectrum is essential for UV/visible detector applications. As radiation traverses a substance, specific wavelengths are absorbed by the molecules, while others are transmitted. This interaction is quantitatively described by the Beer-Lambert Law, which asserts that absorbance is directly proportional to both the concentration of the absorbing species and the distance of radiation through the substance. Mastery of this principle is essential for accurately interpreting spectrophotometric data across various applications, including the use of a UV/visible detector in chemical analysis and medical diagnostics.
Key concepts such as absorbance—the quantity of light absorbed by a sample—are crucial for determining concentration levels, while transmittance refers to the fraction of light that successfully passes through the sample, both of which can be measured using a UV/visible detector to provide insight into its properties. The Beer-Lambert Law serves as a foundational mathematical relationship connecting absorbance to concentration and path length, and is widely utilized in laboratory and clinical settings with the help of a UV/visible detector.
In 2025, the applications of the Beer-Lambert Law extend beyond traditional chemical analysis into pioneering medical diagnostics, utilizing UV/visible detectors. For instance, the calculation of blood oxygen saturation is performed using a UV/visible detector to measure absorbance at specific wavelengths, particularly at the isosbestic points of 520 nm and 546 nm, as highlighted by Anderson et al. This method has demonstrated effectiveness in clinical environments, enhancing the accuracy of physiological assessments through the use of a UV/visible detector. Furthermore, the law's adaptability allows for modifications that account for scattering and fluorescence, which are critical in real-world applications where ideal conditions are rarely met, particularly when using a UV/visible detector. The modified Beer-Lambert Law (MBLL) improves measurement accuracy for UV/visible detector applications by addressing scattering effects, making it particularly useful in complex biological systems.
Understanding the limitations and modifications of the Beer-Lambert Law is equally important, as real-world conditions often violate its basic assumptions. Factors such as light scattering, fluorescence, and the non-homogeneity of samples can lead to inaccuracies in measurements. Therefore, employing modified versions of the law can yield more reliable data, particularly in complex biological systems. This adaptability underscores the law's enduring relevance in both chemical analysis and medical diagnostics, establishing it as a cornerstone of modern analytical techniques.
Identify Different Types of UV/Visible Detectors
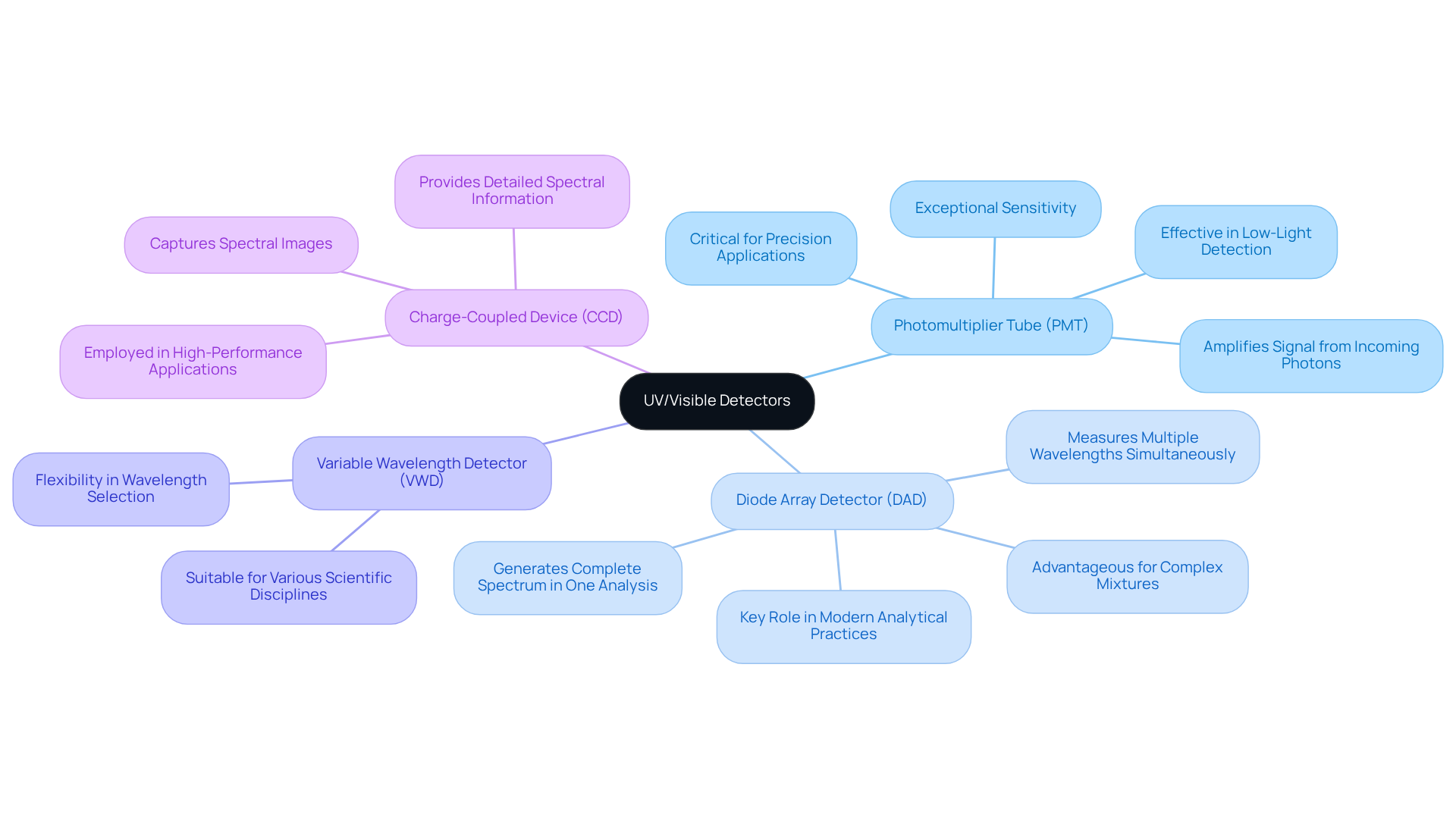
Various types of uv/visible detectors are essential for analytical chemistry, as each offers distinct features and applications.
-
Photomultiplier Tube (PMT): Known for its exceptional sensitivity, the PMT is particularly effective in low-light detection scenarios. By amplifying the signal from incoming photons, PMTs excel at identifying weak signals. This makes them invaluable in applications where precision is critical.
-
Diode Array Detector (DAD): This detector stands out for its ability to measure multiple wavelengths simultaneously, generating a complete spectrum of data in a single analysis. Such capability is especially advantageous when dealing with complex mixtures, allowing for comprehensive characterization of samples. Industry leaders emphasize the importance of DADs in managing these mixtures, highlighting their role in modern analytical practices.
-
Variable Wavelength Detector (VWD): The VWD offers flexibility by enabling users to select specific wavelengths for their analyses. This versatility makes it suitable for a broad spectrum of uses across various scientific disciplines.
-
Charge-Coupled Device (CCD): Similar to DADs, CCDs capture spectral images and are employed in high-performance applications. Their ability to provide detailed spectral information enhances their utility in advanced analytical tasks.
Understanding these sensor types is crucial for selecting the appropriate instrument based on factors such as sensitivity, speed, and the specific attributes of the samples being examined. As noted by Srividya Kailasam, a PhD in analytical chemistry, "Ultraviolet-visible (UV-Vis) spectroscopy is a simple, non-destructive and cost-effective technique that finds applications in fields as diverse as pharmaceutical, food and environmental analysis." This statement underscores the significance of uv/visible detectors in various analytical contexts, reinforcing their relevance in advancing scientific practices.
Set Up and Calibrate UV/Visible Detectors
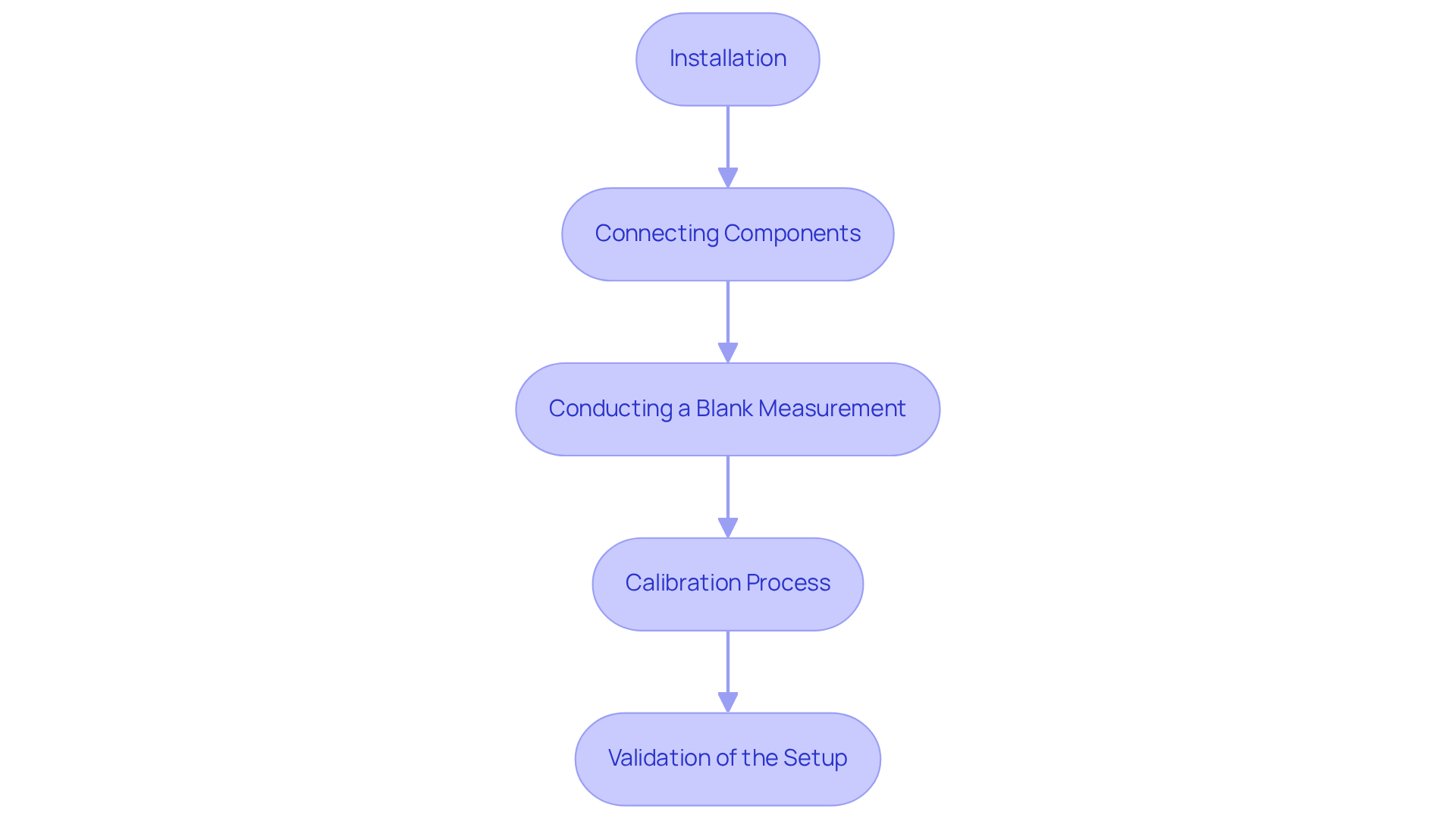
Setting up and calibrating a uv/visible detector is a critical process that involves several key steps essential for accurate measurements.
-
Installation:
Place the device on a stable, level surface, ensuring it is away from direct sunlight and vibrations. It is crucial to verify that the power supply meets the manufacturer's specifications to avoid any operational issues. -
Connecting Components:
Link the sensor to the light source and the specimen holder, ensuring all fittings are secure to prevent leaks, which could compromise the integrity of the measurements. -
Conducting a Blank Measurement:
Measure a blank specimen, typically a solvent, to establish a baseline. This initial step aids in zeroing the instrument, setting the stage for accurate readings. -
Calibration Process:
The calibration process for the uv/visible detector involves using standard solutions, such as potassium dichromate, to adjust the instrument at specific wavelengths. Measure the absorbance of these standards and create a calibration curve, which serves as a reference for future measurements. -
Validation of the Setup:
After calibration, run known samples to ensure the accuracy of the instrument. Regular calibration is not merely a recommendation; it is essential to maintain the integrity of the measurements and should be performed according to the manufacturer's guidelines.
Troubleshoot and Maintain UV/Visible Detectors
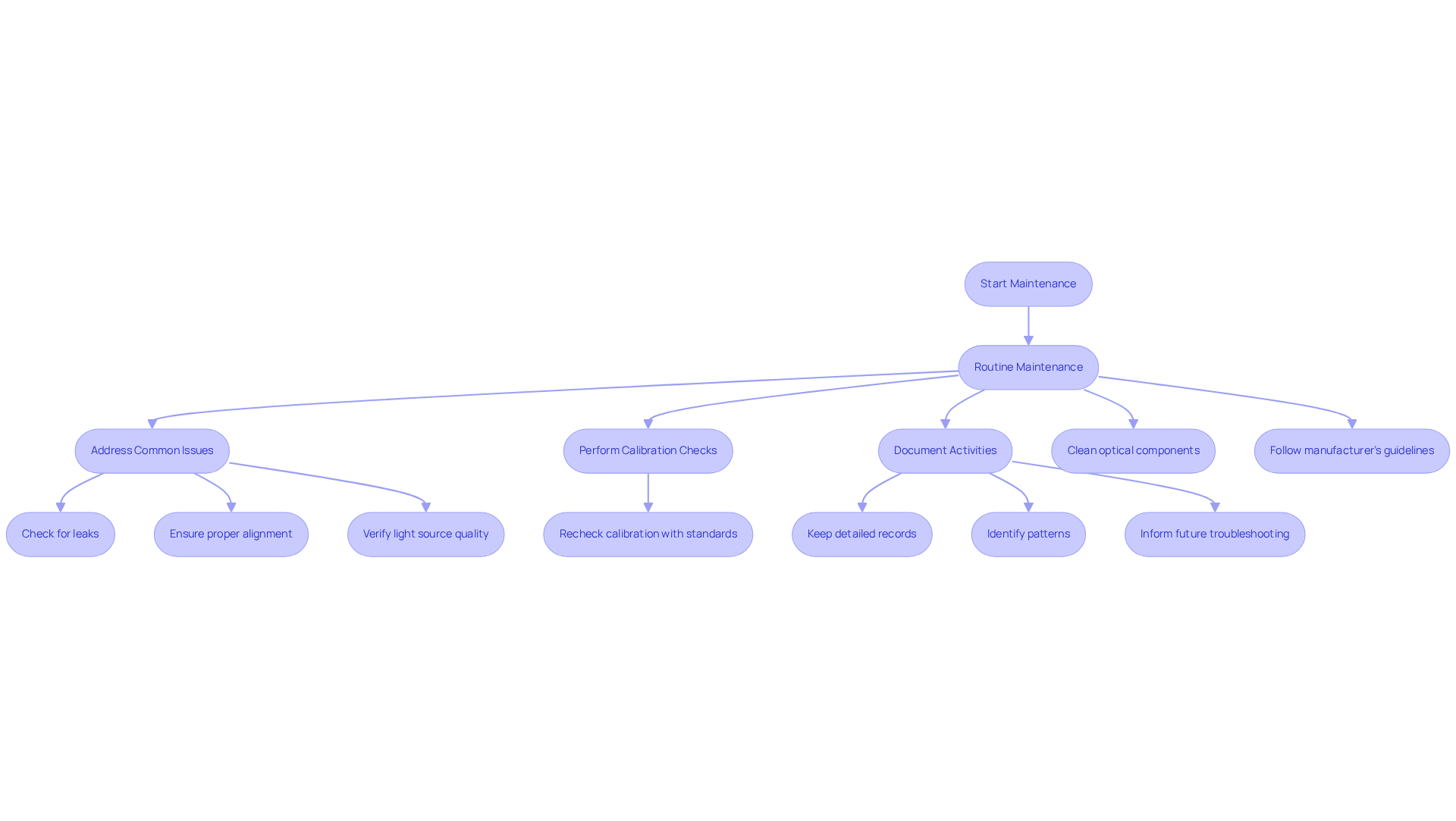
Regular maintenance and troubleshooting are essential to ensure the optimal performance and longevity of UV/visible detectors.
Routine Maintenance: Regular cleaning of optical components—such as lenses and mirrors—as well as the measurement compartment is vital to prevent contamination. Adhering to the manufacturer's guidelines for cleaning procedures will enhance the effectiveness of these efforts.
Common Issues: It is crucial to address common problems, including signal noise, drift, or baseline fluctuations. Checking for leaks, ensuring proper alignment, and verifying the quality of the light source are effective strategies for resolving these issues.
Calibration Checks: Periodically, it is necessary to recheck calibration with standard solutions to maintain accuracy over time. This practice reinforces the reliability of your measurements.
Documentation: Keeping detailed records of maintenance activities, calibration results, and any encountered issues is imperative. This documentation not only helps identify patterns but also informs future troubleshooting efforts.
By following these practices, users can maintain the reliability of their UV/visible detector, ensuring consistent analytical results and fostering confidence in their laboratory processes.
Conclusion
Mastering the setup, calibration, and maintenance of UV/visible detectors is crucial for achieving accurate and reliable analytical results. Understanding the underlying principles of UV/visible detection, particularly the Beer-Lambert Law, provides a solid foundation for interpreting spectrophotometric data across various applications. This knowledge is essential for professionals in fields ranging from chemical analysis to medical diagnostics, where precision is paramount.
The article highlights the different types of UV/visible detectors, including:
- Photomultiplier Tubes
- Diode Array Detectors
- Variable Wavelength Detectors
- Charge-Coupled Devices
Each with unique features suited for specific applications. It emphasizes the importance of proper setup and calibration procedures, detailing necessary steps such as:
- Installation
- Blank measurement
- The creation of calibration curves using standard solutions
Additionally, routine maintenance and troubleshooting practices are outlined to ensure optimal performance and longevity of these instruments.
Ultimately, the effective use of UV/visible detectors not only enhances the accuracy of analytical measurements but also supports advancements in scientific research and medical diagnostics. By adhering to best practices in setup, calibration, and maintenance, professionals can foster confidence in their laboratory processes and contribute to the ongoing evolution of analytical techniques. Embracing these principles will empower users to unlock the full potential of UV/visible spectroscopy in their respective fields.




