Overview
This article serves as a comprehensive guide to mastering Karl Fischer titration, focusing on the fundamental principles, essential equipment, detailed step-by-step procedures, and effective troubleshooting strategies for common issues. By elucidating the chemical reactions involved, the necessary materials, and best practices, this guide underscores the method's critical role in accurately measuring water content across various industries. This accuracy is paramount for ensuring product quality and compliance, thereby reinforcing the significance of high-quality scientific instruments in laboratory settings.
Introduction
Karl Fischer titration is a cornerstone method in analytical chemistry, celebrated for its precision in measuring water content across various industries, from pharmaceuticals to food production. This guide presents a streamlined approach to mastering this essential technique, delineating it into four straightforward steps that guarantee accuracy and reliability.
Despite its widespread application, many practitioners encounter challenges that may compromise results. What are the common pitfalls, and how can they be effectively navigated? Understanding these aspects is crucial for achieving optimal results in your analytical endeavors.
Understand the Principles of Karl Fischer Titration
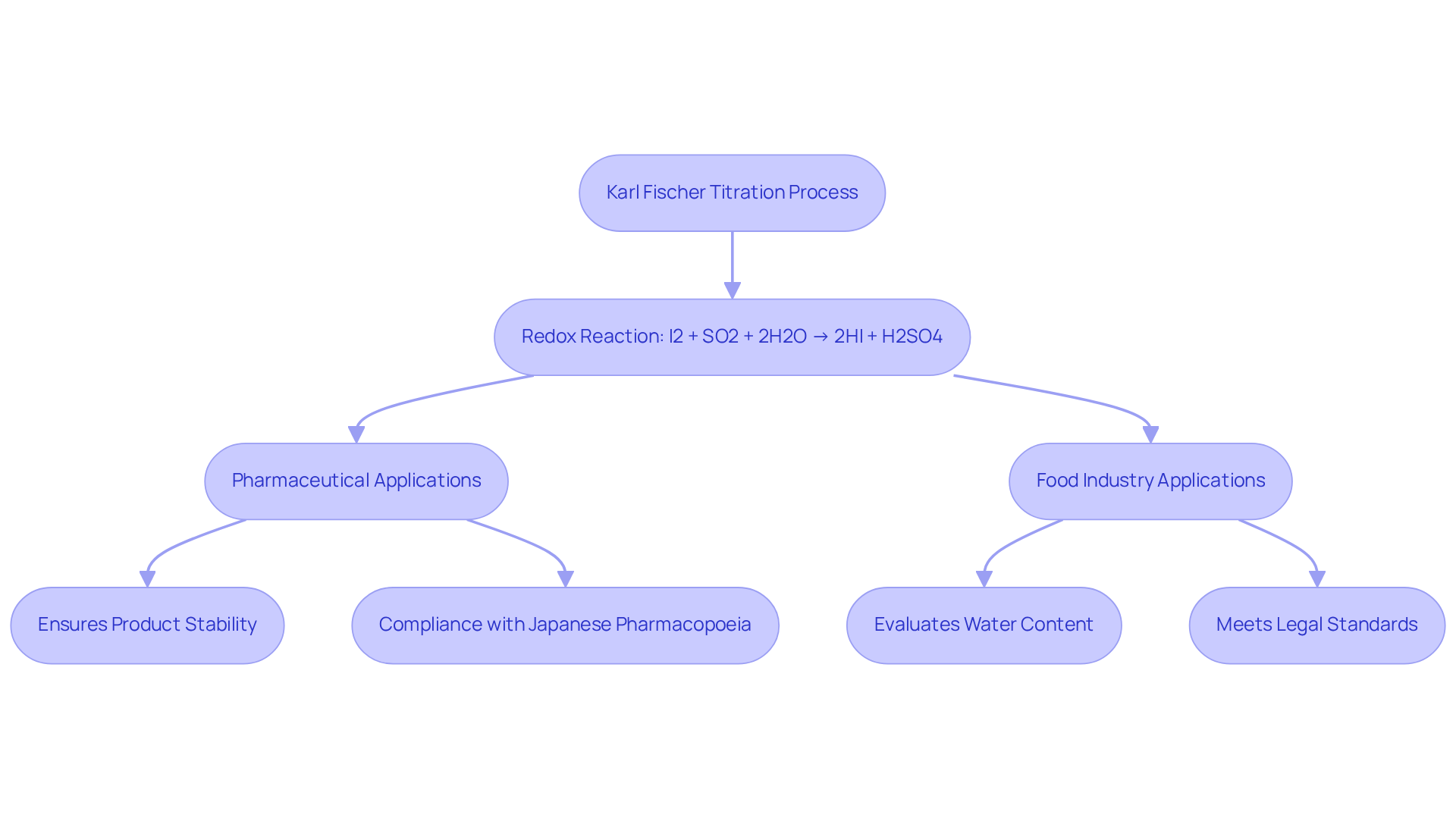
The method serves as a pivotal analytical approach for measuring water content Karl Fischer in various materials, utilizing a redox reaction in which iodine reacts with water in the presence of sulfur dioxide and a base, typically imidazole or pyridine. This reaction can be succinctly summarized as follows:
I2 + SO2 + 2H2O → 2HI + H2SO4
In this process, iodine (I2) is reduced to hydroiodic acid (HI), effectively consuming water. This specificity for water detection highlights the method's suitability across diverse applications, particularly within the pharmaceutical, food, and chemical industries.
Understanding the fundamentals of this method is essential for pharmaceutical applications, where precise water content Karl Fischer assessment is critical for ensuring product stability and effectiveness. The Hiranuma Aquacounter AQV-300 Volumetric and AQ-300 Coulometric Karl Fischer Titrators are meticulously designed for drug and medicine testing, ensuring compliance with the Japanese Pharmacopoeia and incorporating suitability tests for accurate assessments. Recent advancements have significantly enhanced the precision and efficiency of measurement processes, including high-resolution sensors and low-dead-volume burets. These innovations effectively tackle common challenges, such as reagent management and environmental variability, thereby ensuring reliable results.
In the food sector, the method known as water content Karl Fischer is employed to evaluate water content, which directly impacts quality characteristics and compliance with legal standards. The method's adaptability facilitates accurate measurements in complex matrices, ensuring that both polar and nonpolar substances are thoroughly analyzed.
Overall, this method remains the gold standard for moisture analysis, with its applications continuously evolving to meet the demands of various industries.
Gather Required Equipment and Reagents
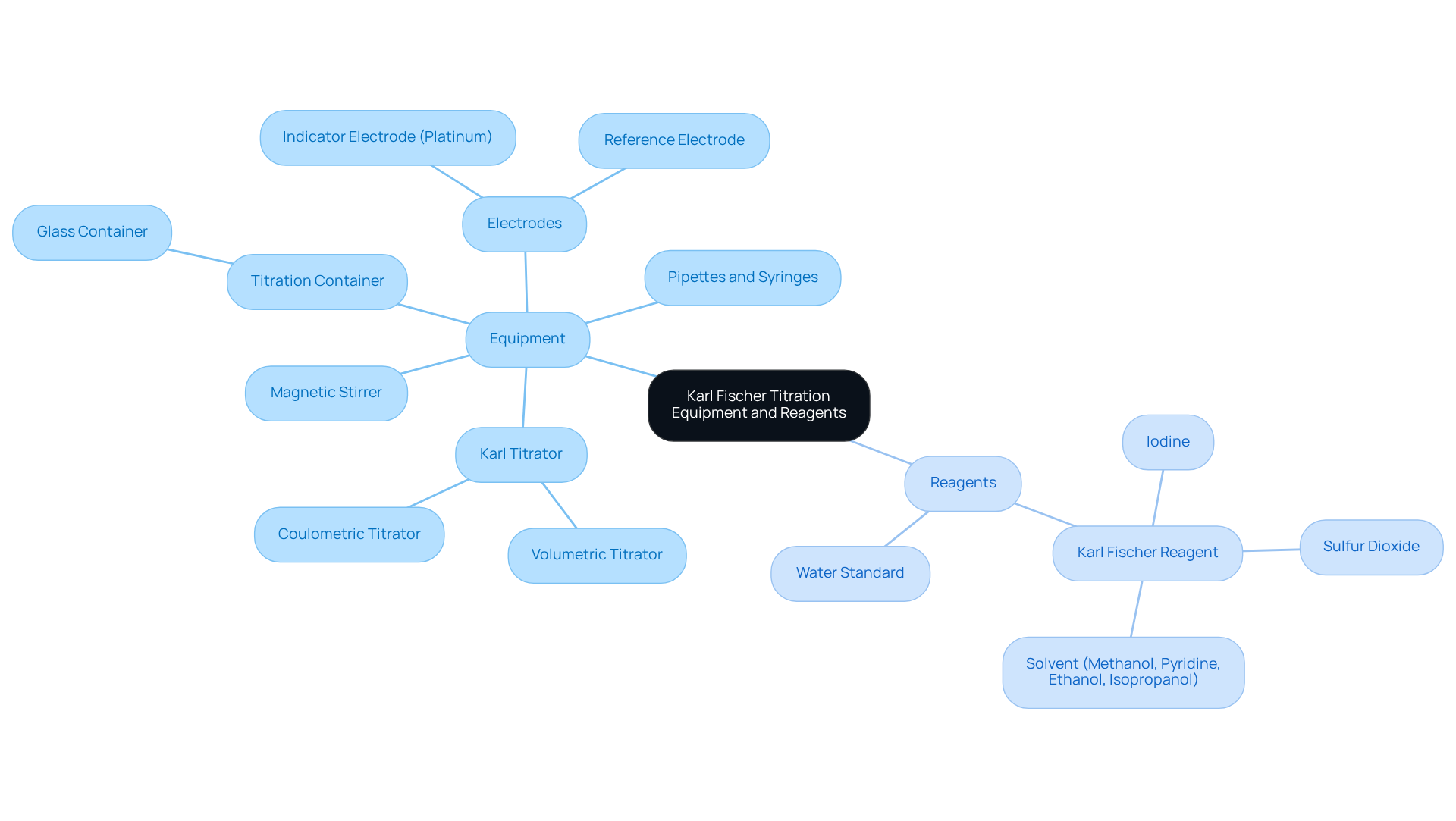
To successfully perform Karl Fischer titration, it is essential to gather the following equipment and reagents:
- Karl Titrator: Select either volumetric or coulometric titrators based on the moisture content range of your samples and the required accuracy for your analysis.
- Titration Container: A glass container capable of withstanding the substances involved in the procedure is necessary.
- Electrodes: Your setup should include a suitable indicator electrode, typically a platinum electrode, along with a reference electrode.
- Reagents:
- Karl Fischer Reagent: This reagent typically comprises iodine, sulfur dioxide, and a solvent such as methanol, pyridine, ethanol, or isopropanol, all of which are crucial for the titration reaction.
- Water Standard: A known water standard is vital for calibration, ensuring accurate results. Regular calibration using certified water standards is essential for maintaining measurement accuracy in water content Karl Fischer analysis.
- Pipettes and Syringes: These tools are necessary for precise measurement of samples and reagents.
- Magnetic Stirrer: This device is crucial for ensuring comprehensive mixing during the process, which is essential for obtaining reliable results.
Gathering these materials in advance will streamline the titration process and enhance accuracy. Laboratory supervisors emphasize that the selection of reagents significantly impacts the reliability of moisture assessments, particularly in relation to water content Karl Fischer. Many have observed that utilizing high-quality reagents from the Karl Fischer method can yield more consistent outcomes in determining water content. In fact, statistics indicate that over 70% of pharmaceutical laboratories employ specific titrators for moisture analysis, underscoring their importance in ensuring product quality and compliance. However, one must consider the operational complexities of the method, including the necessity for skilled technicians and potential interferences from certain compounds, which can affect result accuracy.
Follow the Step-by-Step Titration Procedure
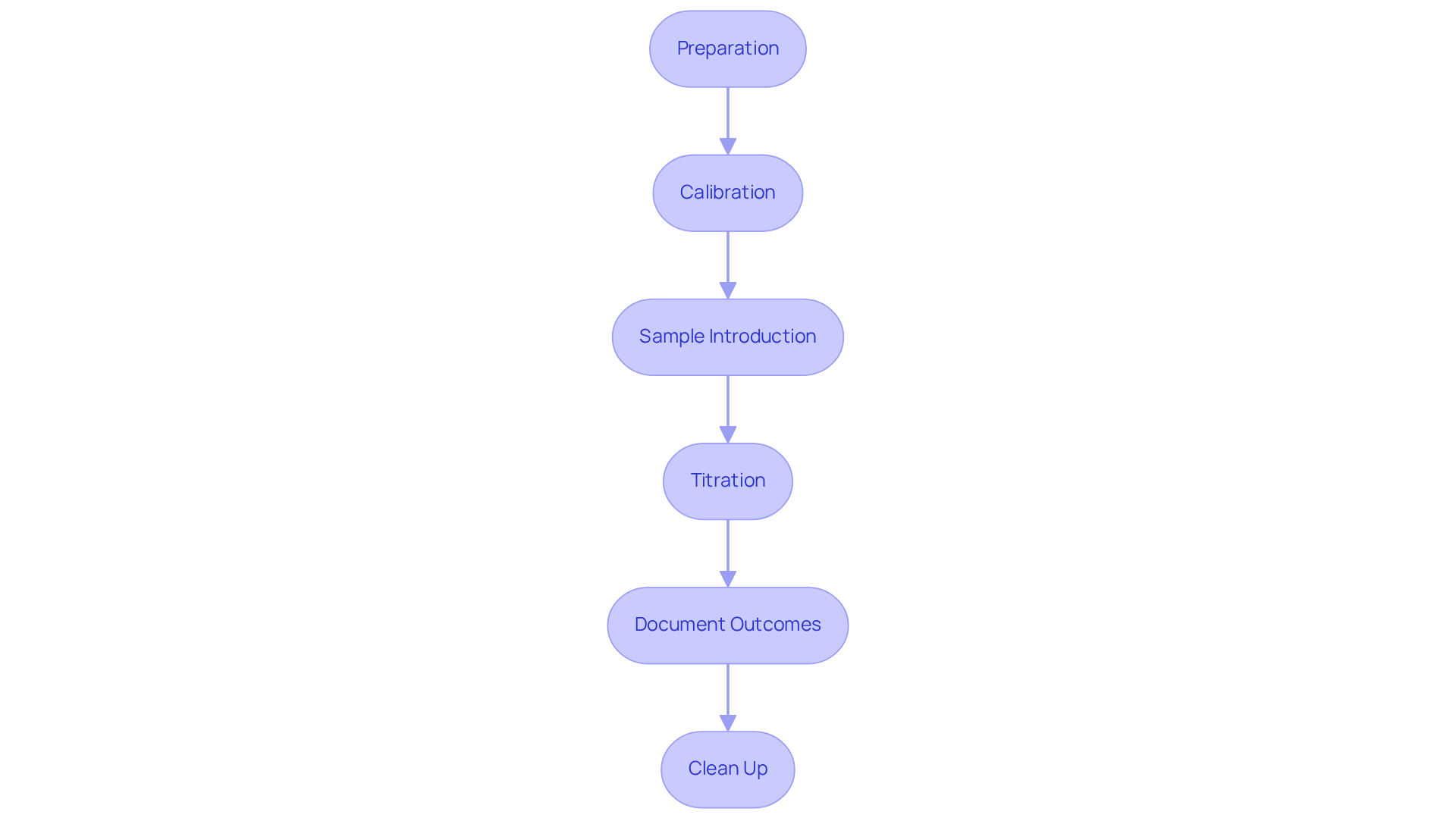
To perform Karl Fischer titration effectively, it is essential to adhere to the following steps:
-
Preparation: Begin by meticulously cleaning and calibrating all equipment. Set up the titrator according to the manufacturer's specifications to avoid contamination. Maintaining storage temperatures between 4°C and 10°C is crucial, as it improves reagent stability, which directly impacts the accuracy of your results.
-
Calibration: Calibrate the titrator using a certified water standard. This step is vital for achieving accurate results; improper calibration can lead to significant errors in determining water content Karl Fischer.
-
Sample Introduction: Weigh an exact quantity of the sample, typically ranging from 0.1 to 1 g, and place it into the reaction vessel. For solid samples, ensure they are finely powdered to facilitate dissolution and enhance measurement accuracy.
-
Titration: Initiate the titration process. The titrator will automatically dispense the Karl Fischer solution until the endpoint is reached, which is determined by a change in potential measured by the electrodes, thus allowing for the measurement of water content Karl Fischer. Modern titrators often incorporate features that track chemical quality and issue notifications for outdated substances, ensuring dependable results. Coulometric methods are particularly suitable for trace levels as low as 1 ppm, while volumetric methods are appropriate for samples with water levels from 100 ppm to 100%.
-
Document Outcomes: After completing the process, carefully note the quantity of substance utilized. This value is crucial for calculating the water content karl fischer in your sample.
-
Clean Up: Thoroughly clean all equipment post-titration to prevent contamination in future analyses. Proper storage of Karl's reagents in tightly sealed containers can also extend their usability and maintain accuracy.
By following these steps, you will ensure accurate and reproducible results in your water content Karl Fischer determination, minimizing common mistakes such as atmospheric moisture interference and improper sample handling. As emphasized by industry specialists, sustaining ideal conditions and adhering to best practices is critical for successful analytical procedures.
Troubleshoot Common Issues in Karl Fischer Titration
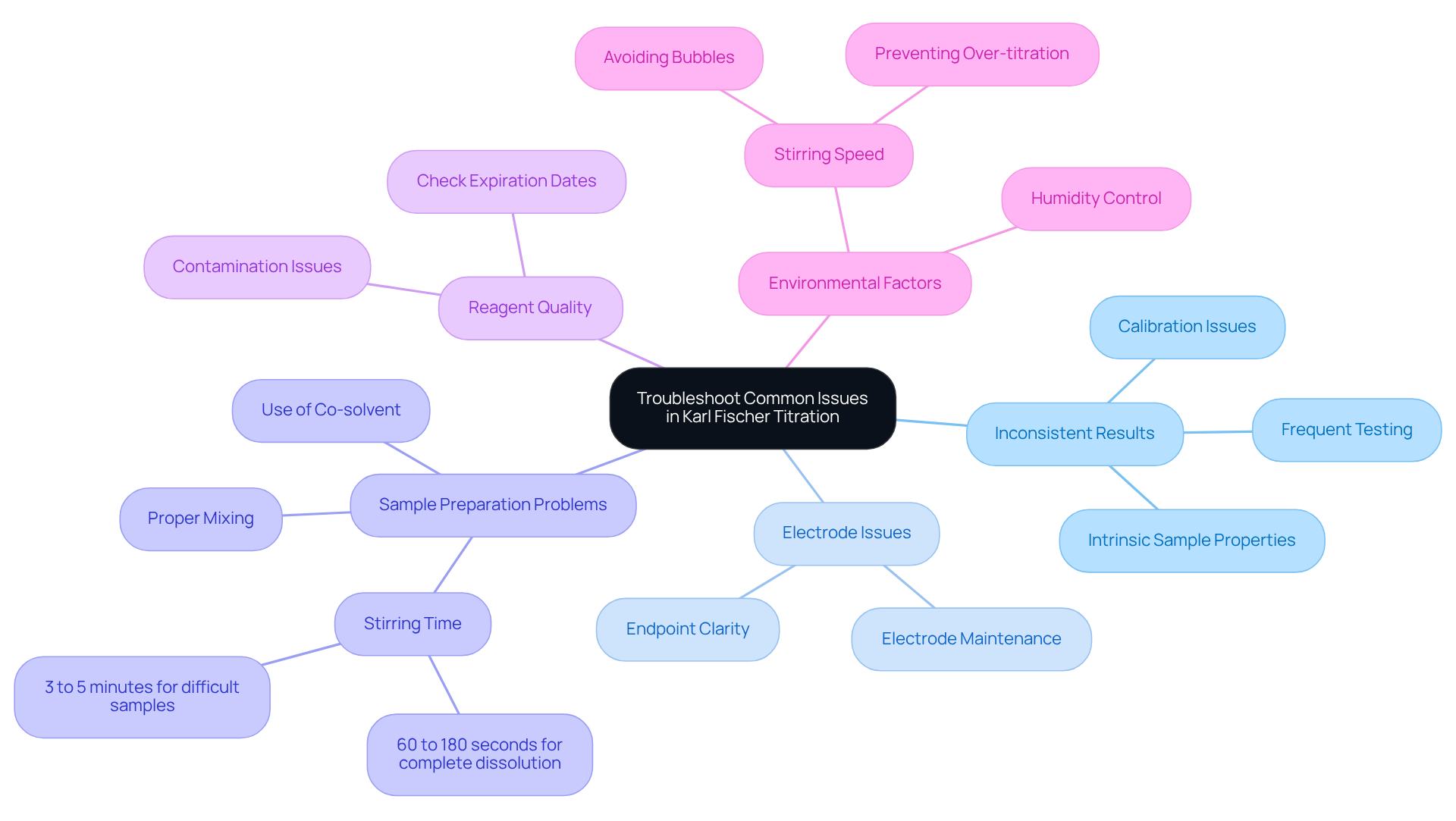
When employing the Karl Fischer titration method, several typical challenges related to water content Karl Fischer may arise. It is crucial to address these issues effectively to enhance your results:
-
Inconsistent Results: Variability in results can frequently be attributed to calibration issues. Ensure that your titrator is properly calibrated, and that the water standard used for calibration is both fresh and precisely measured. Conducting frequent checks can help maintain consistency. Additionally, variations in moisture testing results may stem from the intrinsic properties of test samples; therefore, consider running multiple tests to minimize these effects.
-
Electrode Issues: A lack of clarity at the endpoint may signal problems with the electrodes. Inspect them for cleanliness and functionality, and clean them according to the manufacturer's guidelines. Regular maintenance is essential to prevent electrode-related discrepancies.
-
Sample Preparation Problems: Proper sample preparation is vital. Solid samples should be finely powdered and thoroughly mixed with the solvent to ensure complete dissolution. If samples are challenging to dissolve, consider using a co-solvent. Inadequate mixing can lead to incomplete reactions and skewed results. For samples that dissolve completely, the recommended stirring time is between 60 to 180 seconds, while those that do not dissolve should be stirred for 3 to 5 minutes.
-
Reagent Quality: If the timing of the analysis exceeds expectations, evaluate the quality and expiration date of your Karl Fischer chemicals. Utilizing old or contaminated reagents can significantly impact accuracy; thus, always verify their condition before use.
-
Environmental Factors: High humidity levels can disrupt analysis results. Conduct measurements in a controlled environment to reduce moisture interference, ensuring more reliable outcomes. Additionally, be mindful of stirring speed; improper stirring can create bubbles or lead to over-titration, adversely affecting accuracy.
By proactively addressing these potential issues, you can significantly improve the reliability and accuracy of your results in measuring water content Karl Fischer. As Michael Margreth, a senior product specialist at Metrohm, emphasizes, understanding these common problems and their solutions is key to enhancing the accuracy and efficiency of moisture analysis.
Conclusion
Mastering Karl Fischer titration is essential for accurately determining water content in various materials, particularly within industries such as pharmaceuticals and food production. This method, noted for its specificity and reliability, employs a redox reaction to detect water, thereby ensuring compliance with quality standards and preserving product integrity.
The article elucidated the fundamental principles of Karl Fischer titration, detailing the necessary equipment and reagents, a comprehensive step-by-step procedure, and common troubleshooting techniques. Each of these components plays a critical role in achieving precise measurements. From proper calibration and sample preparation to addressing environmental factors and ensuring reagent quality, every aspect significantly contributes to the accuracy of results obtained through this analytical technique.
In summary, understanding and implementing best practices in Karl Fischer titration not only enhances measurement accuracy but also underscores the importance of meticulousness in laboratory environments. Whether in pharmaceuticals, food production, or chemical analysis, the significance of accurately determining moisture content cannot be overstated. Adopting these practices ensures adherence to industry standards and ultimately supports the development of high-quality products.




