Overview
This article delves into the essential mastery of laboratory pH meter calibration and the best practices necessary for ensuring accurate pH measurements. It underscores the critical nature of regular calibration using certified buffer solutions, proper storage techniques, and routine maintenance. These practices are vital for enhancing the reliability and longevity of pH meters, which in turn is crucial for achieving consistent results across various scientific applications. By adhering to these best practices, laboratory professionals can significantly improve their measurement accuracy, thereby reinforcing the integrity of their scientific work.
Introduction
Understanding the intricacies of pH measurement is essential for anyone involved in laboratory work, as it plays a critical role in determining the acidity or alkalinity of solutions. Mastery of pH meters not only ensures precise experimental outcomes but also enhances the reliability of results across various scientific fields.
However, many researchers encounter challenges with calibration and maintenance, leading to potential inaccuracies that could compromise their findings. How can laboratory professionals effectively navigate these complexities to harness the full potential of their pH meters?
This question underscores the need for a deeper exploration into best practices and strategies that can empower researchers to optimize their use of this vital instrument.
Explore the Fundamentals of pH Measurement
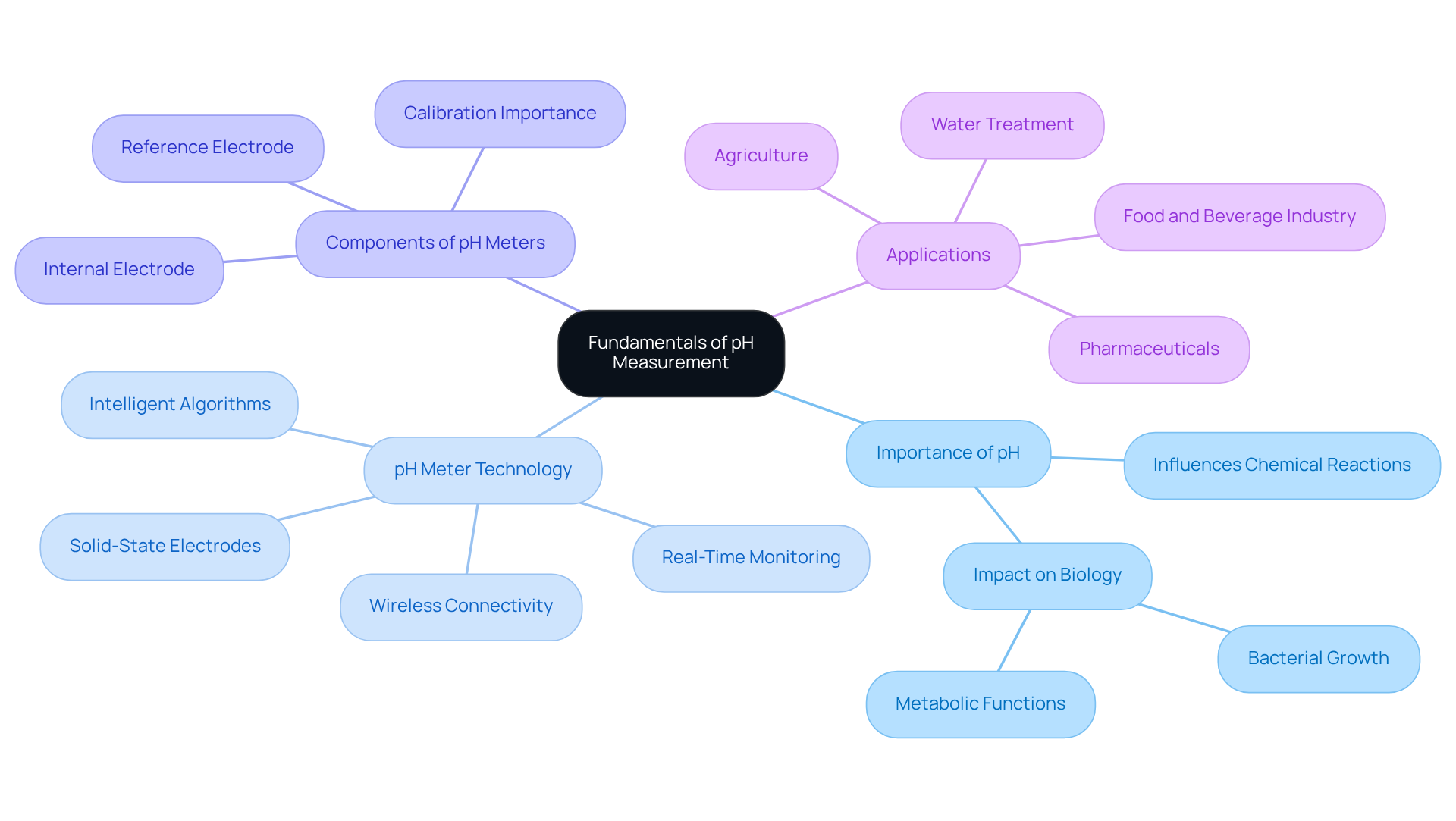
The laboratory pH meter is a cornerstone of laboratory analysis, serving as a key indicator of a mixture's acidity or alkalinity. The pH scale, ranging from 0 to 14, categorizes solutions as acidic (below 7), neutral (7), or basic (above 7). This understanding is crucial across various scientific disciplines, including chemistry, biology, and environmental science, as pH significantly influences chemical reactions and biological processes. For instance, different bacteria thrive at specific pH levels, making pH monitoring vital in experimental setups.
Laboratory pH meters are the primary instruments for measuring this parameter, providing precise readings that are critical for experimental accuracy. Recent advancements in pH measurement technology, such as solid-state electrodes and integrated sensors allowing for simultaneous measurement of pH, temperature, and other parameters, have further enhanced their utility. Furthermore, the introduction of intelligent algorithms enables real-time monitoring and predictive analytics, transforming pH devices into powerful tools for process optimization.
Understanding the components of a laboratory pH meter, such as the internal electrode and reference liquid, is essential for effective usage and maintenance. Models like the Starter 300 and Starter 400M offer features such as data storage and wireless capabilities, making them suitable for various applications. Proper calibration with suitable buffer liquids ensures dependable measurements, enabling researchers to accurately evaluate the acidity or alkalinity of their samples. As pH levels can affect the behavior of substances in solutions, maintaining precise control over pH with a laboratory pH meter is vital for achieving consistent and reproducible results in laboratory analyses. Moreover, pH instruments are widely utilized in sectors such as food and beverage, pharmaceuticals, and water treatment, underscoring their significance in ensuring safety and quality.
Master Calibration Techniques for Accurate pH Readings
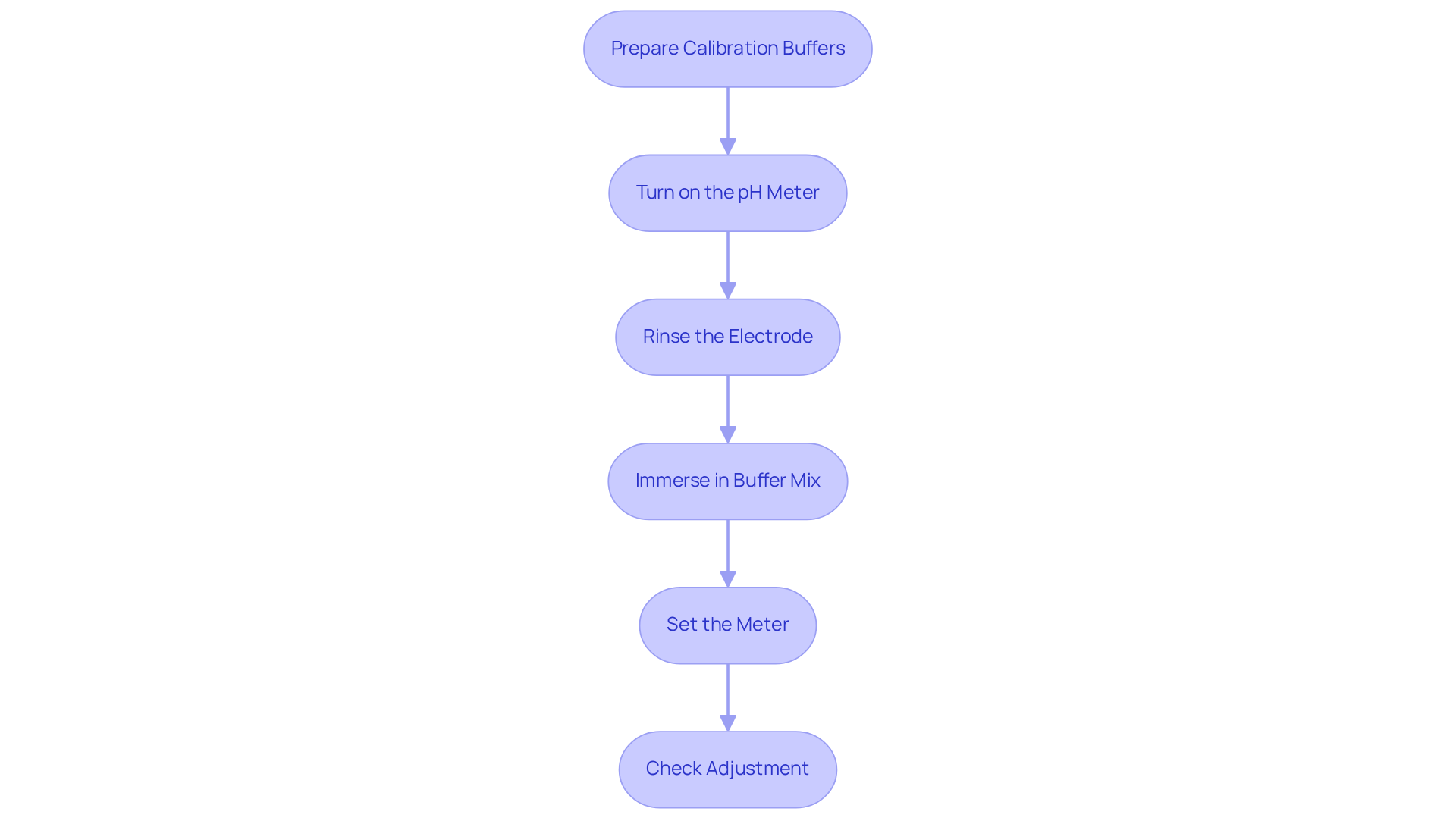
To achieve accurate pH readings, regular calibration of the pH meter is essential. Calibration must be conducted using certified standard buffer liquids, typically at pH values of 4.00, 7.00, and 10.00. The following step-by-step guide outlines the calibration process:
- Prepare the Calibration Buffers: Ensure that the buffer mixtures are fresh and at room temperature. Utilize high-quality, certified buffer mixtures for optimal outcomes.
- Turn on the pH Meter: Allow the meter to warm up for a few minutes before calibration.
- Rinse the Electrode: Clean the pH electrode with distilled water to prevent contamination, ensuring that no residues from prior mixtures affect the measurement.
- Immerse in Buffer Mix: Place the electrode in the first buffer mix (usually pH 7.00) and allow the reading to stabilize.
- Set the Meter: Adjust the meter to read the correct pH value of the buffer. Repeat this process with the other buffer mixtures.
- Check Adjustment: After the adjustment, verify the accuracy by measuring the pH of a known solution. If the reading is off, recalibrate.
Regular calibration, ideally before each use, ensures that the pH meter remains accurate and reliable. It is advisable to calibrate at least once a week or prior to significant tests to maintain precision in readings. Laboratories that utilize a laboratory pH meter and follow these practices frequently report improved accuracy, which is essential for experimental integrity and product quality across various applications, including pharmaceuticals and food safety. By employing certified buffer solutions and adhering to appropriate adjustment techniques, laboratories can significantly diminish the risk of errors in essential measurements. As stated in the Supmea pH Sensor User Manual, 'Regular and precise adjustments ensure accurate and dependable data.' Furthermore, calibration practices in research laboratories demonstrate the importance of a three-point calibration method to enhance accuracy across a range of values.
Implement Best Practices for pH Meter Usage and Maintenance
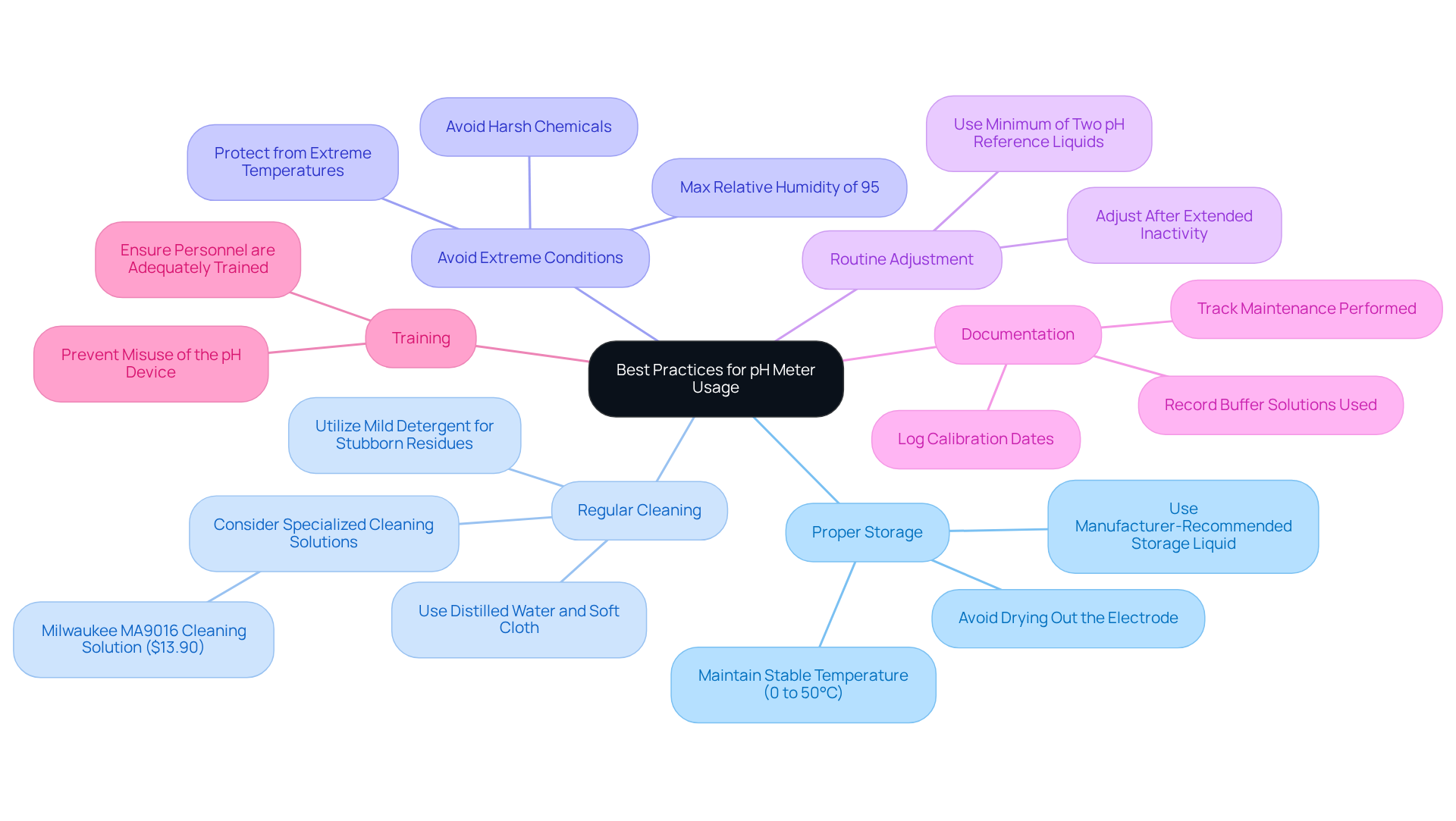
To maximize the performance and longevity of your pH meter, it is essential to adhere to best practices that ensure reliability and accuracy in measurements.
-
Proper Storage: Always keep the pH electrode in a manufacturer-recommended storage liquid. Avoid allowing it to dry out, as this can lead to irreversible damage to the electrode. For optimal results, maintain the storage method at a stable temperature, ideally between 0 to 50°C. Neglecting proper storage can significantly shorten the lifespan of the electrode.
-
Regular Cleaning: Clean the electrode frequently using distilled water and a soft cloth. For more stubborn residues, utilize a mild detergent or a specialized cleaning solution, such as the Milwaukee MA9016 Cleaning Solution, priced at $13.90, which is specifically designed to maintain electrode integrity.
-
Avoid Extreme Conditions: Protect the pH device from extreme temperatures and harsh chemicals, as exposure can compromise its accuracy and shorten its lifespan. Ensure that the operating environment maintains a maximum relative humidity of 95% to prevent moisture-related issues.
-
Routine Adjustment: Regular adjustments are crucial for preserving accuracy, particularly following extended periods of inactivity. Routine adjustment of pH meters is vital to guarantee precise readings and should be conducted frequently, especially before important assessments. Adjustment should be carried out with a minimum of two distinct pH reference liquids to ensure dependable readings throughout the anticipated measurement range.
-
Documentation: Maintain a log of calibration dates, buffer solutions used, and any maintenance performed. This practice aids in monitoring the device's performance over time and ensures that all required procedures are adhered to consistently.
-
Training: Ensure that all personnel using the pH device are adequately trained in its operation and maintenance. This training helps prevent misuse and encourages consistent, precise outcomes in laboratory evaluations. As industry specialists emphasize, 'Whether you’re a researcher, a farmer, or a quality control manager, a dependable pH device is an invaluable asset.'
By following these best practices, laboratory personnel can ensure that their pH meters remain reliable tools for precise measurements, ultimately enhancing the quality of their scientific work.
Conclusion
Mastering the use of a laboratory pH meter is essential for achieving reliable and accurate measurements across various scientific fields. The significance of pH and its impact on chemical reactions and biological processes underscores the necessity of proper calibration and maintenance. By implementing effective calibration techniques and adhering to best practices, researchers can ensure their pH meters function optimally, leading to consistent and reproducible results.
This article highlights critical aspects such as:
- The importance of regular calibration using certified buffer solutions
- Proper storage and cleaning of the electrode
- Routine adjustments to maintain accuracy
Key practices, including documenting calibration dates and training personnel, further enhance the reliability of pH measurements. These insights serve to underline the role of pH meters in diverse applications, from pharmaceuticals to environmental monitoring, where precision is crucial.
To maximize the value of pH measurements in laboratory settings, prioritizing calibration and maintenance is imperative. By fostering a culture of diligence around these practices, laboratories can enhance the quality of their scientific work and contribute to broader advancements in research and industry. Embracing these best practices ensures that pH meters remain reliable tools for accurate measurements, ultimately supporting the integrity of experimental outcomes and product safety.




