Overview
Mastering chromatogram peak analysis in pharmaceutical labs is crucial for achieving accurate and reliable results. Key concepts such as:
- Retention time
- Summit area
- Summit form
- Resolution
must be understood to excel in this field. By adhering to best practices in:
- Equipment selection
- Mobile phase preparation
- Systematic analysis steps
laboratories can significantly optimize their chromatographic processes. This approach not only addresses common challenges like peak tailing and resolution issues but also enhances overall efficiency and reliability in laboratory outcomes.
Introduction
Understanding the intricacies of chromatogram peak analysis is essential for pharmaceutical labs striving for precision in their results. Each peak in a chromatogram reveals vital information about the composition of a sample; therefore, mastering peak evaluation techniques can significantly enhance the reliability of analytical outcomes. Yet, numerous challenges—such as peak tailing, splitting, and resolution issues—persist.
How can laboratories ensure they extract the most accurate data possible? This article delves into the fundamentals, technical setups, and step-by-step processes necessary for effective chromatogram peak analysis, equipping professionals with the knowledge to navigate these complexities and elevate their analytical capabilities.
Fundamentals of Chromatogram Peak Analysis
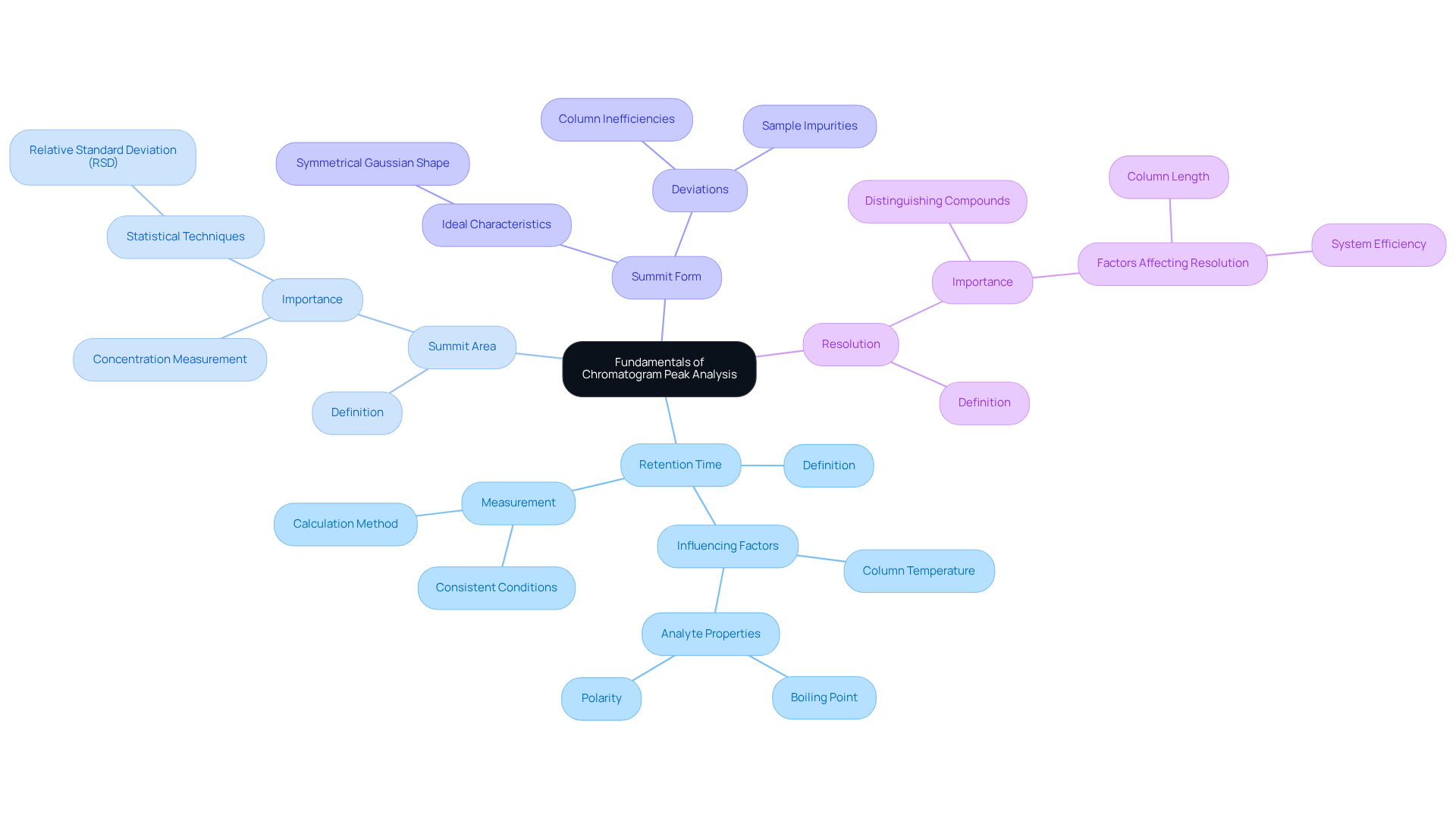
Chromatogram signal evaluation is paramount in chromatographic methods, particularly within pharmaceutical laboratories. Each chromatogram peak corresponds to a component of a mixture as it elutes from the chromatographic system. Understanding key concepts is essential:
- Retention Time: This denotes the duration a compound takes to traverse the column to the detector, unique for each compound under consistent conditions. Variations in can stem from factors such as column temperature and the properties of the analytes, including boiling point and polarity. For example, compounds with lower boiling points typically exhibit shorter retention times due to their higher vapor pressures.
- Summit Area: The area beneath a summit is directly proportional to the concentration of the substance present in the sample. Larger area sizes indicate higher concentrations, making precise measurement crucial for quantification. Statistical techniques, such as the relative standard deviation (RSD), are often employed to assess the reliability of area measurements, ensuring precision in analytical outcomes.
- Summit Form: Ideally, summits should present a symmetrical, Gaussian shape. Deviations from this ideal can indicate potential issues, such as column inefficiencies or sample impurities. For instance, ghost signals observed in ultra-high-performance liquid chromatography (UHPLC) can vary from 1.5 to 2.5 minutes, suggesting possible contamination or system irregularities.
- Resolution: This refers to the ability to distinguish between two neighboring summits. High resolution is critical for accurate quantification and identification of compounds, especially in complex mixtures. Various factors, including column length and the efficiency of the chromatographic system, can affect resolution.
Understanding these fundamental elements of chromatogram peak evaluation is vital for enhancing separation efficiency and ensuring the reliability of results in pharmaceutical applications. By maintaining consistent experimental conditions and leveraging advanced technologies, laboratories can enhance their analytical capabilities and contribute significantly to the advancement of research and healthcare.
Technical Setup for Chromatogram Analysis
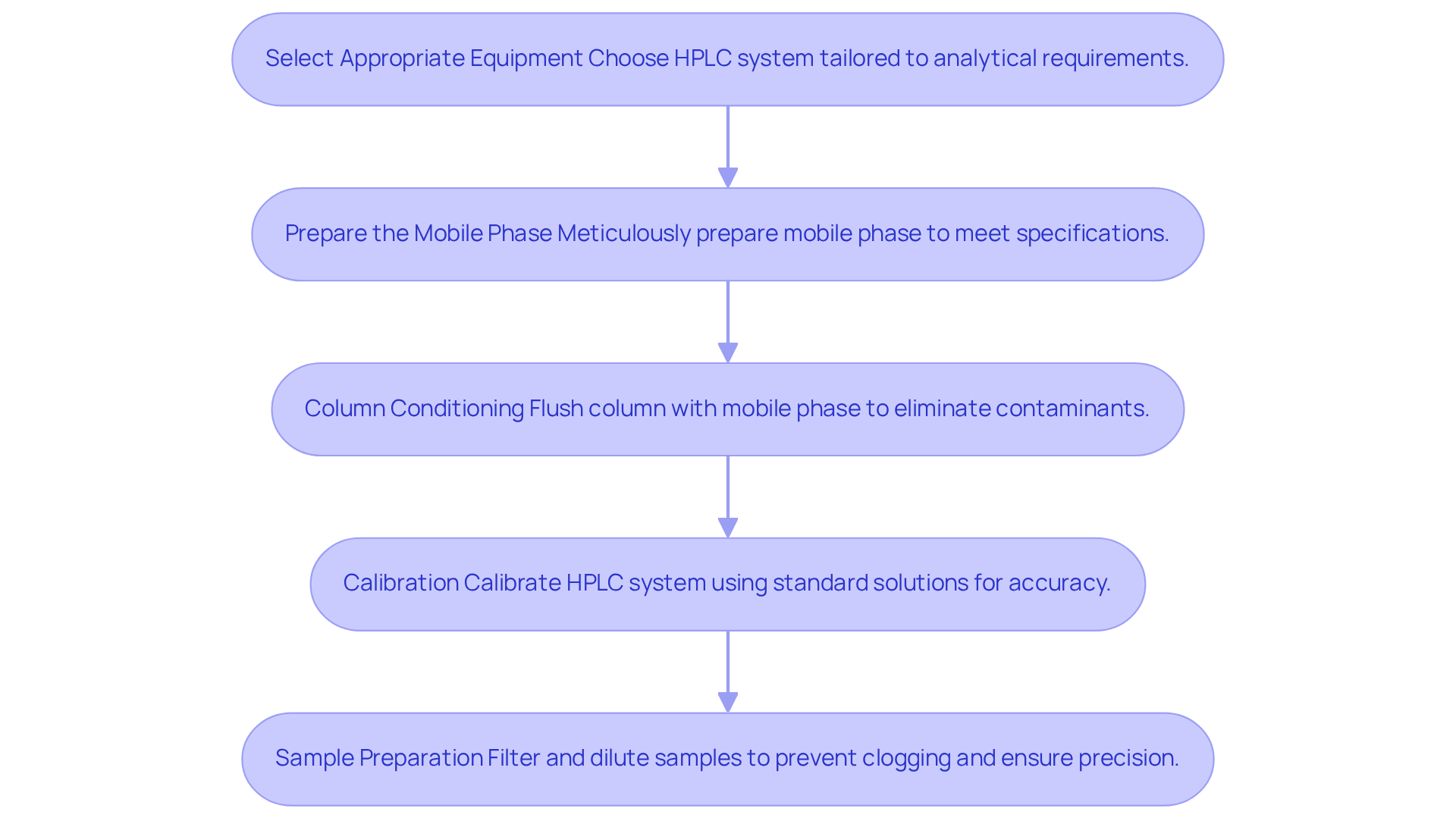
Setting up for chromatogram peak analysis involves several critical steps that are essential for achieving accurate and reliable results.
- Select Appropriate Equipment: It is imperative to choose a high-performance liquid chromatography (HPLC) system tailored to your analytical requirements. This selection includes that align with the specific characteristics of the samples being analyzed. For instance, employing gradient elution methods can significantly enhance separation efficiency by altering the mobile phase composition during the examination. While lower flow rates can improve resolution, they also increase analysis time, presenting an important trade-off to consider.
- Prepare the Mobile Phase: The mobile phase must be meticulously prepared to meet method specifications, with careful attention to pH and ionic strength. Each component should be measured separately before mixing to ensure accuracy. For example, achieving a 70% organic mobile phase necessitates precise measurements of 300 mL of water and 700 mL of organic solvent. Furthermore, utilizing high-quality gradient grade solvents minimizes contamination risks and extends the durability of chromatographic devices. It is crucial to measure pH before adding organic solvents, as inadequate pH measurement can lead to inaccurate results. As Genevieve Hodson states, "Liquid Chromatography is reliant upon an accurately made mobile phase."
- Column Conditioning: Properly conditioning the column by flushing it with the mobile phase is vital to eliminate contaminants and equilibrate the stationary phase. This step is crucial for maintaining consistent performance and ensuring reliable results.
- Calibration: Calibrating the HPLC system using standard solutions is necessary to guarantee accurate measurements. This process includes setting the flow rate and adjusting the detector response to align with the expected analyte concentrations. Regular calibration helps to mitigate common issues arising from improper mobile phase preparation, which can lead to inaccurate retention times.
- Sample Preparation: Preparing samples by filtering and diluting them as necessary prevents clogging of the system and ensures precise results. Employing a 0.45 µm filter is recommended to remove suspended impurities from the mobile phase, thereby enhancing the quality of the chromatogram peak in the chromatographic analysis. It is also important to recognize that expired additives can introduce unfamiliar signals in chromatograms, further highlighting the need for careful reagent management.
By adhering to these best practices, laboratories can optimize their chromatographic processes, ensuring robust and reproducible results in pharmaceutical applications.
Step-by-Step Guide to Analyzing Chromatogram Peaks
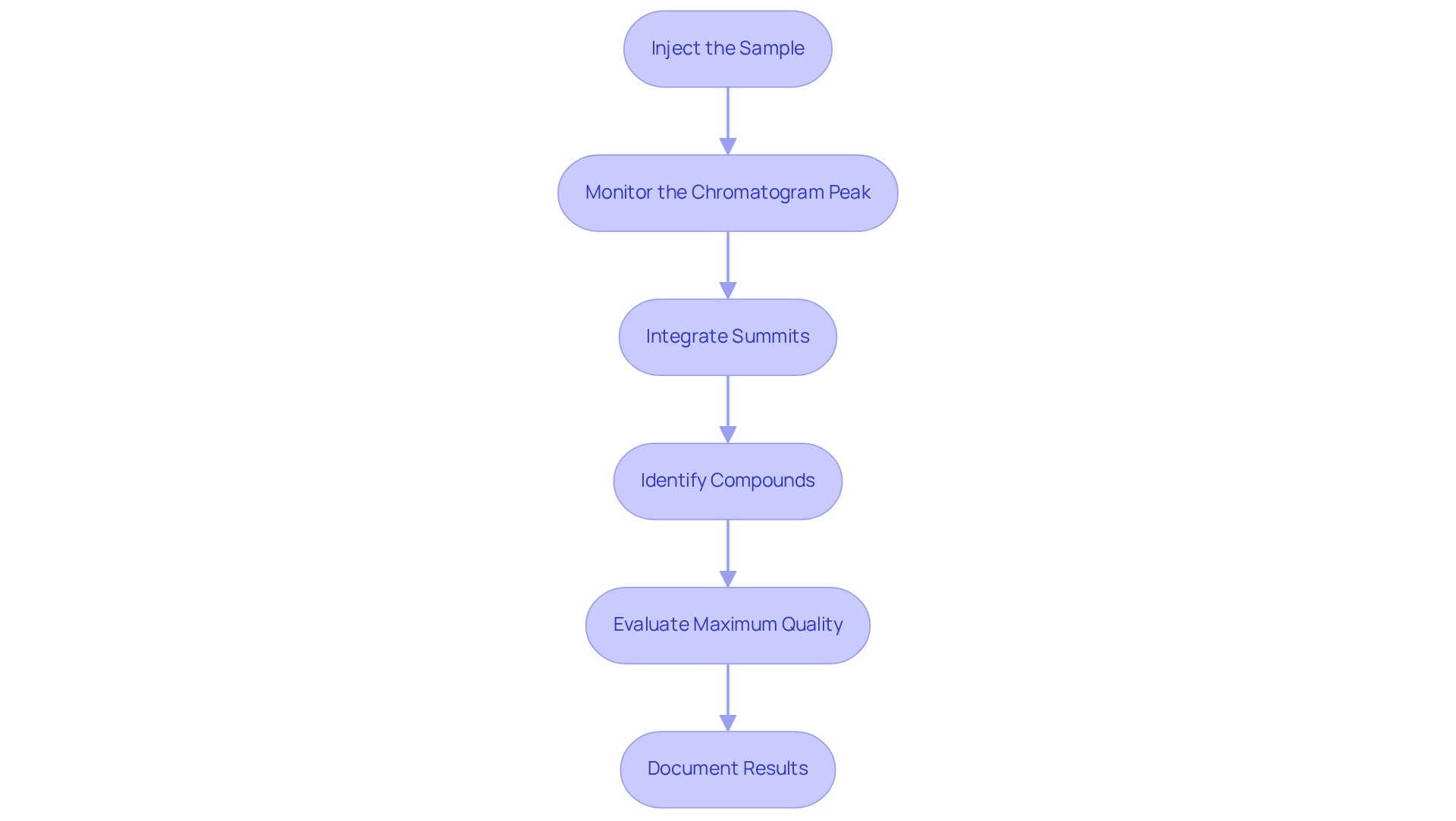
To effectively analyze chromatogram peaks in HPLC, it is imperative to follow these essential steps:
- Inject the Sample: Employ either an autosampler or manual injection to introduce the prepared sample into the HPLC system. This ensures consistent and accurate sample delivery, which is crucial for reliable results.
- Monitor the chromatogram peak: Continuously observe the chromatogram as it develops. Pay close attention to retention times and signal shapes, which are critical for accurate analysis. Notably, inter-day precision coefficients of variation (CV%) for HPLC methods typically range between 0.20% and 0.61%, indicating reliable performance across different runs.
- Integrate Summits: Utilize chromatography software to combine the summits, calculating the area beneath each summit for quantification. This step is essential for determining the concentration of analytes in the specimen, ensuring precise measurement.
- Identify Compounds: Compare the retention durations and area measurements with those of established standards to accurately determine the compounds present in the material. This comparison is vital for confirming the identity of the analytes, which reinforces the integrity of the chromatogram peak analysis.
- Evaluate Maximum Quality: Assess the symmetry and clarity of the summits. Look for signs of tailing, fronting, or splitting, which may indicate potential issues with the evaluation, such as column overload or sample degradation. As emphasized in the verification of for Ga-68-DOTATATE, ensuring optimal quality is essential for precise results.
- Document Results: Carefully note all findings, including chromatograms, area measurements, and any irregularities observed during the examination. Comprehensive documentation is crucial for maintaining quality control and ensuring reproducibility in laboratory results. As Akira Kotani highlights, repeatability evaluation is crucial to achieving dependable and accurate quantitative outcomes in pharmaceutical examination.
Challenges and Solutions in Peak Analysis
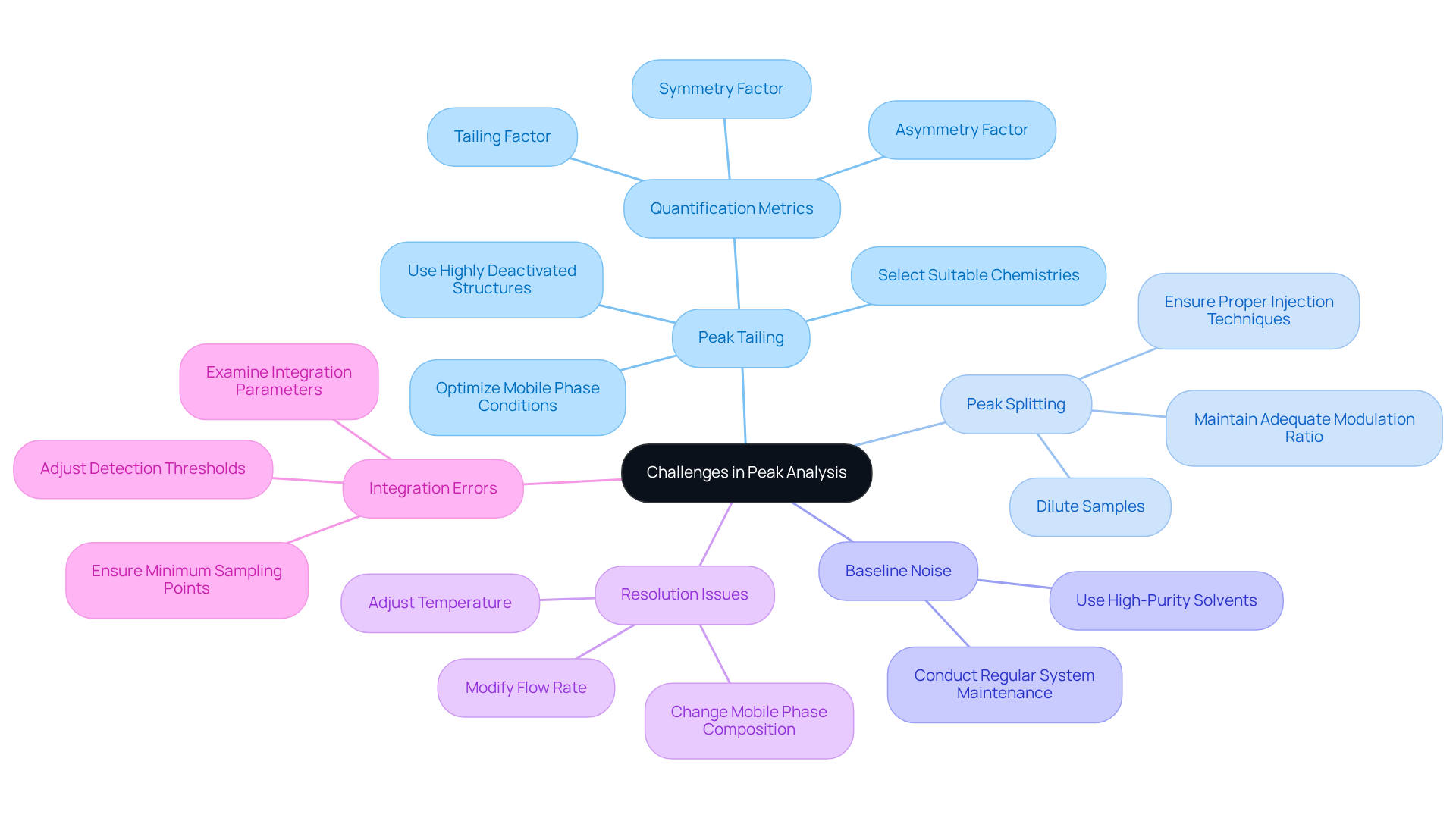
Addressing common challenges in chromatogram peak analysis is critical for achieving accurate results in laboratory settings.
- Peak Tailing: This issue often arises from interactions between the analyte and the stationary phase, particularly with compounds that possess amine or basic functional groups. To alleviate maximum tailing, optimizing mobile phase conditions and selecting suitable chemistries are essential. For instance, utilizing highly deactivated structures can significantly enhance the shape of the signal by reducing secondary interactions. Studies indicate that peak tailing can be quantified using metrics such as the Tailing Factor, Asymmetry Factor, and Symmetry Factor, with ideal values reflecting perfect peak symmetry.
- Peak Splitting: Peak splitting may occur due to column overload or inadequate specimen preparation. To mitigate this, it is advisable to dilute samples and ensure proper injection techniques. Furthermore, maintaining an adequate modulation ratio in comprehensive two-dimensional liquid chromatography (LC×LC) is vital for preserving first dimension resolution and achieving reliable quantification. Research suggests that the optimum quantitative precision in 2D separations is achieved with modulation ratios around two.
- Baseline Noise: Variations in the baseline can obscure high point detection, complicating evaluation. Employing high-purity solvents and conducting regular system maintenance can minimize contamination and enhance baseline stability. Studies have demonstrated that baseline fluctuations significantly affect precision in LC×LC analysis.
- Resolution Issues: Poor resolution may lead to overlapping signals, undermining analytical accuracy. Adjusting factors such as temperature, flow rate, or can greatly improve the separation and resolution of the chromatogram peak. The implementation of robust methods and consistent column performance is essential for reliable quality control in laboratories.
- Integration Errors: Incorrect integration settings can result in flawed quantification of values. It is crucial to examine integration parameters and adjust detection thresholds as needed to ensure dependable results. For example, ensuring a minimum of three sampling points enhances the accuracy of peak fitting, particularly when utilizing Gaussian methods. The average precision of the Gaussian method in recent studies was found to be 5.8%, compared to 4.2% for the moments method, underscoring the importance of employing the correct integration approach.
By implementing these strategies, laboratories can significantly enhance the reliability and accuracy of their chromatographic analyses, ultimately leading to improved outcomes in pharmaceutical testing.
Conclusion
Mastering chromatogram peak analysis is paramount for pharmaceutical laboratories striving for precision and reliability in their analytical processes. This tutorial has delved into the essential concepts of chromatogram evaluation, encompassing:
- Retention time
- Summit area
- Summit form
- Resolution
Each of these components is vital in ensuring accurate results, highlighting the necessity of meticulous attention to detail in chromatographic methods.
The article further delineated the technical setup required for effective chromatogram analysis, stressing the importance of:
- Selecting appropriate equipment
- Preparing the mobile phase
- Ensuring proper calibration and sample preparation
Adhering to a structured step-by-step guide for analyzing chromatogram peaks enables laboratories to optimize their methodologies while addressing prevalent challenges such as peak tailing, splitting, and baseline noise. By implementing strategic solutions, labs can significantly enhance the reliability of their analytical outcomes.
In conclusion, the importance of mastering chromatogram peak analysis cannot be overstated in the pharmaceutical sector. As laboratories pursue excellence, adopting advanced techniques and best practices will not only improve analytical capabilities but also contribute to the broader advancement of research and healthcare. Continuous learning and adaptation to emerging trends in chromatogram analysis will ensure that pharmaceutical labs remain at the forefront of innovation and accuracy in their testing methodologies.




