Overview
The primary focus of this article is to master chromatographic plate techniques, a crucial aspect for achieving optimal results in analytical chemistry. Selecting the appropriate type of chromatographic plate is paramount; it sets the foundation for effective analysis. By adhering to precise procedures for usage, one can significantly enhance separation efficiency and ensure data integrity. This is particularly vital in applications such as pharmaceutical analysis and quality control, where accuracy is non-negotiable. Addressing common challenges faced in these processes further bolsters the reliability of results, underscoring the importance of expertise in this field.
Introduction
Chromatographic plates serve as the cornerstone of modern analytical chemistry, essential for the effective separation of complex mixtures. Their performance is influenced by critical factors such as the type of stationary phase and the number of theoretical layers, which directly impact resolution and accuracy of results. As industries like pharmaceuticals and environmental monitoring increasingly demand precision, practitioners must adeptly navigate these techniques to optimize their outcomes. To address common challenges and elevate the effectiveness of chromatographic plates, it is vital to explore strategies that enhance the reliability of analytical results.
Define Chromatographic Plates and Their Role in Separation Techniques
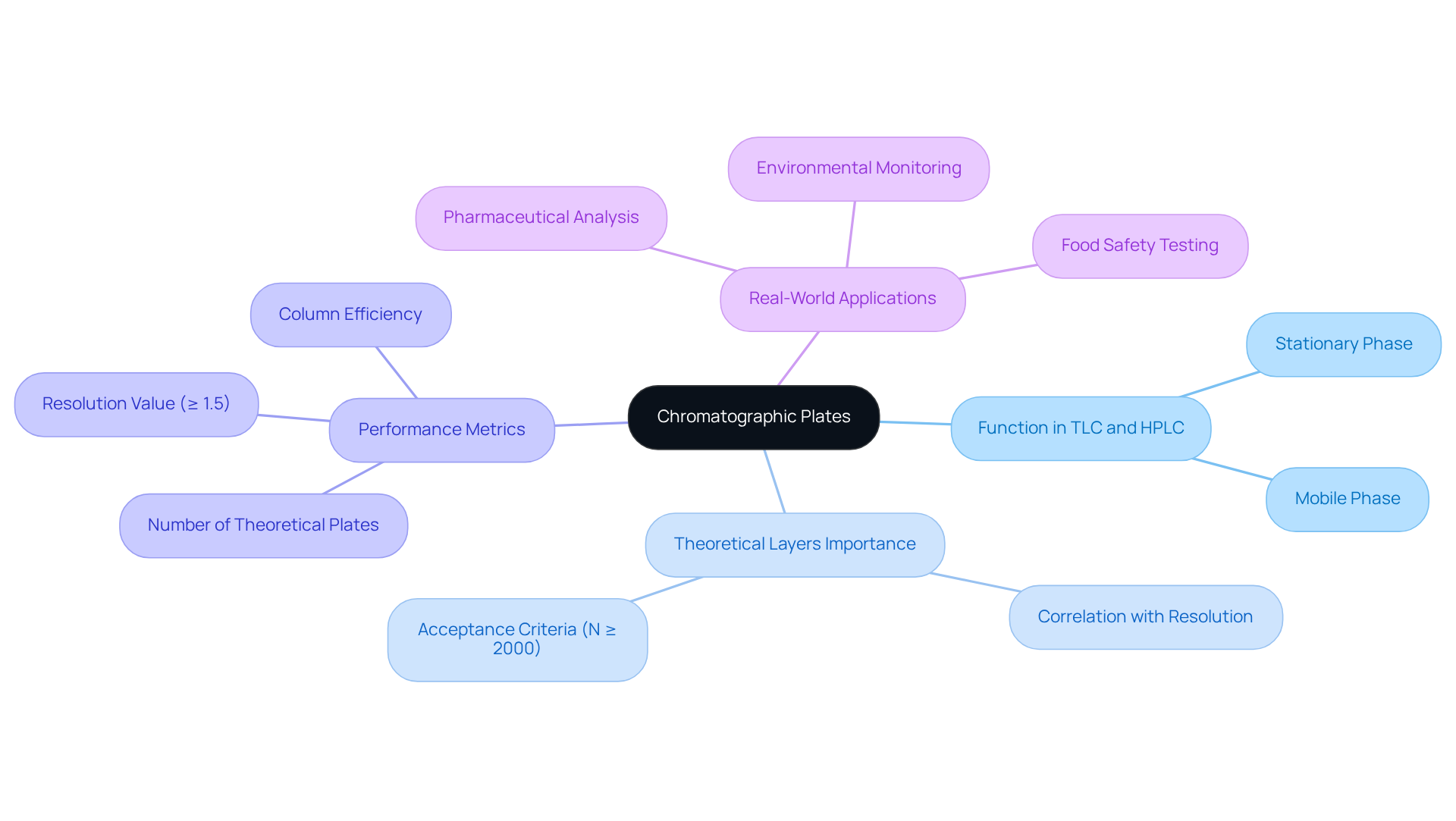
Chromatographic plates serve as vital components in various separation techniques, notably in thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC). These discs consist of a stationary phase affixed to a support material, which facilitates the separation of mixture components based on their distinct affinities for both the stationary and mobile phases.
The efficacy of a chromatographic surface is measured by the number of theoretical layers, signifying its ability to separate compounds effectively. A higher number of theoretical layers correlates with sharper peaks and improved resolution, which are essential for achieving precise analytical results. In HPLC assessments, the acceptance criteria typically require N to be at least 2000, while a resolution value of ≥ 1.5 is crucial for the baseline resolution of two peaks with equal areas.
Recent trends indicate a growing emphasis on optimizing these components to enhance performance in HPLC applications, ensuring compliance with regulatory standards and bolstering data integrity. Real-world instances demonstrate that careful consideration of chromatographic surface characteristics can profoundly impact outcomes in pharmaceutical analysis, environmental monitoring, and food safety testing.
As J. Calvin Giddings aptly notes, 'the quantity of theoretical stages is an indicator of the 'quality' of the column,' underscoring the importance of these metrics in chromatography. Understanding the role of these components is vital for refining separation methods and achieving reliable results when using a chromatographic plate in analytical chemistry.
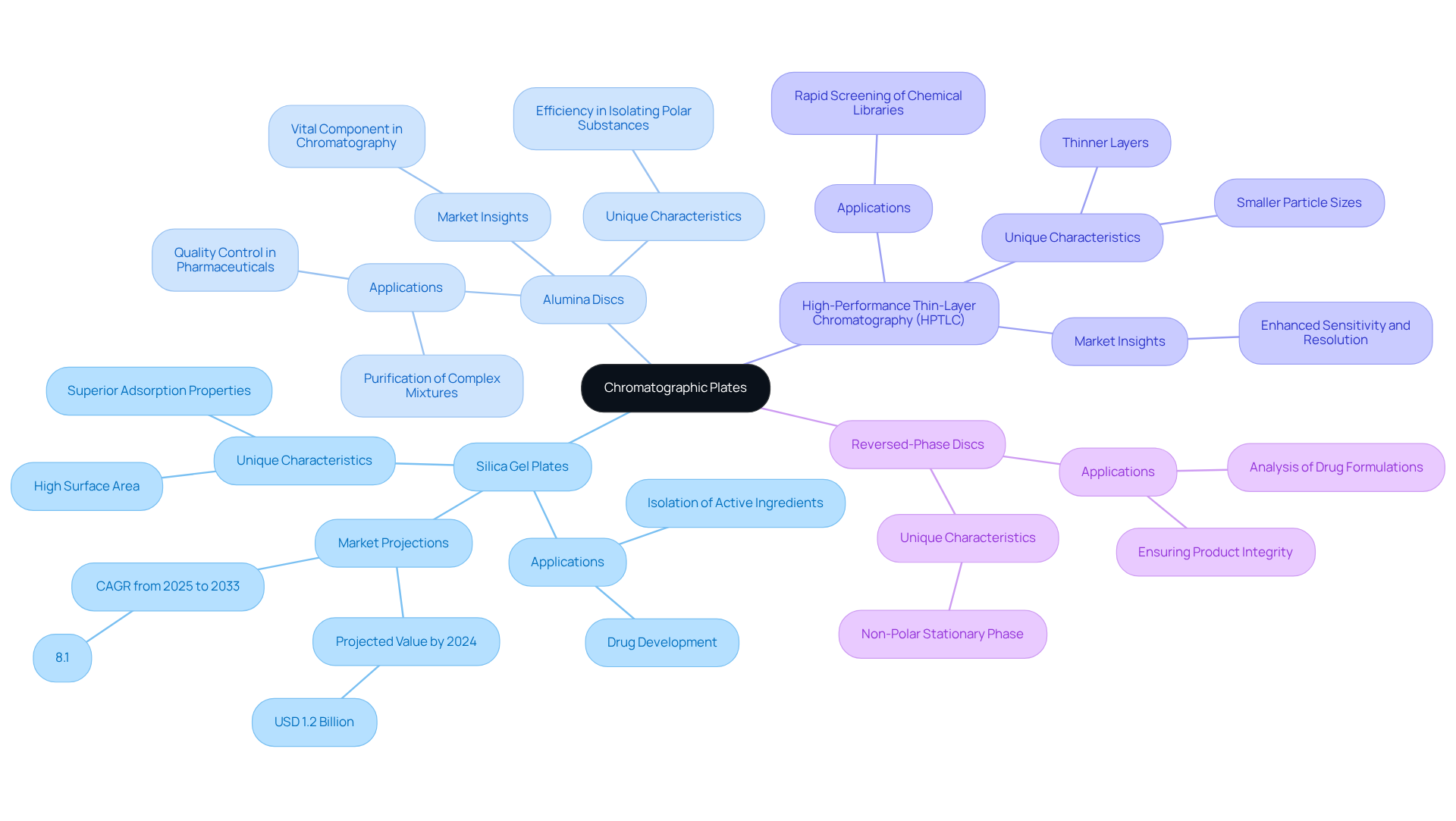
Explore Different Types of Chromatographic Plates and Their Applications
Chromatographic plates serve as essential tools in analytical chemistry, tailored for distinct applications. The most prevalent types include:
-
Silica Gel Plates: Extensively utilized in thin-layer chromatography (TLC), these plates excel in the separation of non-volatile compounds. Their high surface area and superior adsorption properties render the chromatographic plate ideal for a variety of applications, particularly in drug development, where it facilitates the isolation of active pharmaceutical ingredients. The market for silica gel sheets is projected to reach a value of USD 1.2 billion by 2024, with an anticipated annual growth rate of approximately 8.1% from 2025 to 2033.
-
Alumina Discs: Known for their efficiency in isolating polar substances, alumina discs are frequently employed in preparative separation techniques. Their unique characteristics enable the effective purification of complex mixtures, making them invaluable in pharmaceutical laboratories for quality control and compound evaluation. Although specific market share statistics for alumina sheets are less frequently reported, they remain a vital component in the chromatography landscape.
-
High-Performance Thin-Layer Chromatography (HPTLC): HPTLC surfaces, which feature smaller particle sizes and thinner layers, are designed as a chromatographic plate to provide higher resolution and expedited analysis. This advancement is particularly advantageous in drug development, where the use of a chromatographic plate allows for rapid screening of chemical libraries. Recent innovations in HPTLC technology have enhanced the sensitivity and resolution of the chromatographic plate, further improving their utility across various applications.
-
Reversed-Phase Discs: Designed for the separation of hydrophobic compounds, these discs utilize a non-polar stationary phase. They are commonly employed in pharmaceutical applications to analyze drug formulations and ensure product integrity.
The market for silica gel sheets is expected to grow significantly, driven by increasing demand in the pharmaceutical industry, where they play a crucial role in drug development and quality assurance. Recent advancements in silica gel technology, including the development of high-performance and specialized surfaces, enhance their applicability across various domains, including environmental monitoring and food safety testing. As the industry progresses, the integration of automation and modern detection technologies continues to elevate the performance and sensitivity of chromatographic techniques.
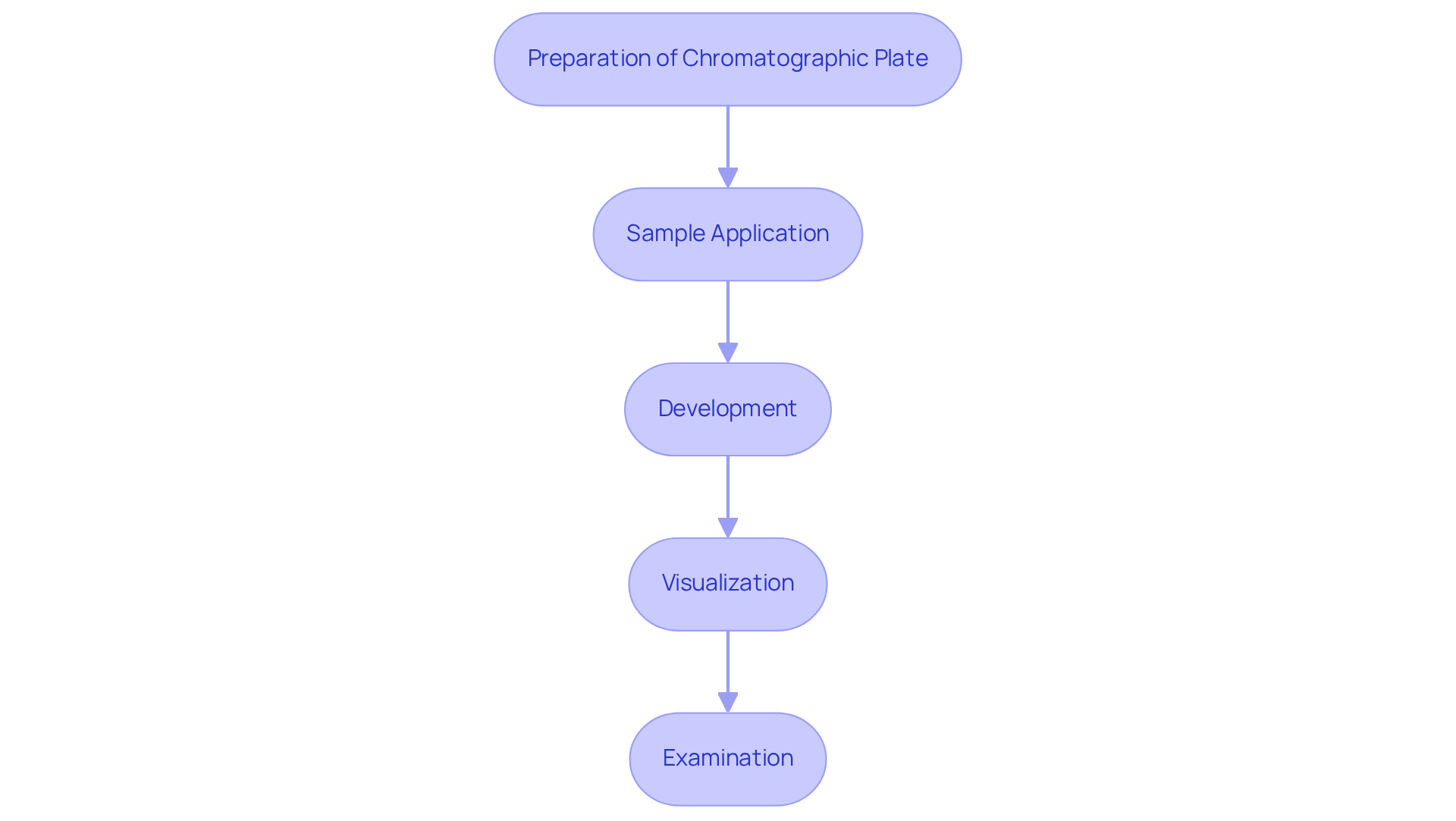
Implement Step-by-Step Procedures for Using Chromatographic Plates
To effectively utilize chromatographic plates, it is essential to follow these step-by-step procedures:
-
Preparation of the chromatographic plate: Begin by thoroughly cleaning the chromatographic surface to eliminate any contaminants. For the chromatographic plate used in thin-layer chromatography (TLC), it is advisable to pre-wash it with an appropriate solvent to ensure optimal performance.
-
Sample Application: Employ a capillary tube or micropipette to apply small, controlled drops of the sample solution onto the baseline of the surface. Each spot should have a diameter of approximately 2 mm, maintaining an even spacing of at least 5 mm between the spots to prevent overlap during development.
-
Development: Place the dish in a developing chamber filled with a suitable solvent (mobile phase). It is crucial that the solvent does not initially contact the sample spots, allowing for proper separation. Cease the chromatography when the solvent level is between halfway and 0.5 cm from the top of the surface to achieve optimal results.
-
Visualization: After the solvent front has progressed adequately, remove the plate and allow it to dry. Utilize UV light or staining techniques to effectively visualize the components that are separated on the chromatographic plate. For colorless substances, alternative visualization techniques may be necessary.
-
Examination: Measure the distance traveled by each component and calculate the retention factor (Rf) for quantitative evaluation. This step is vital for comparing results across different samples and ensuring accurate identification of compounds. The calculated percentage recovery in a study varies between 79.38% and 131.62%, underscoring the importance of precision in sample analysis.
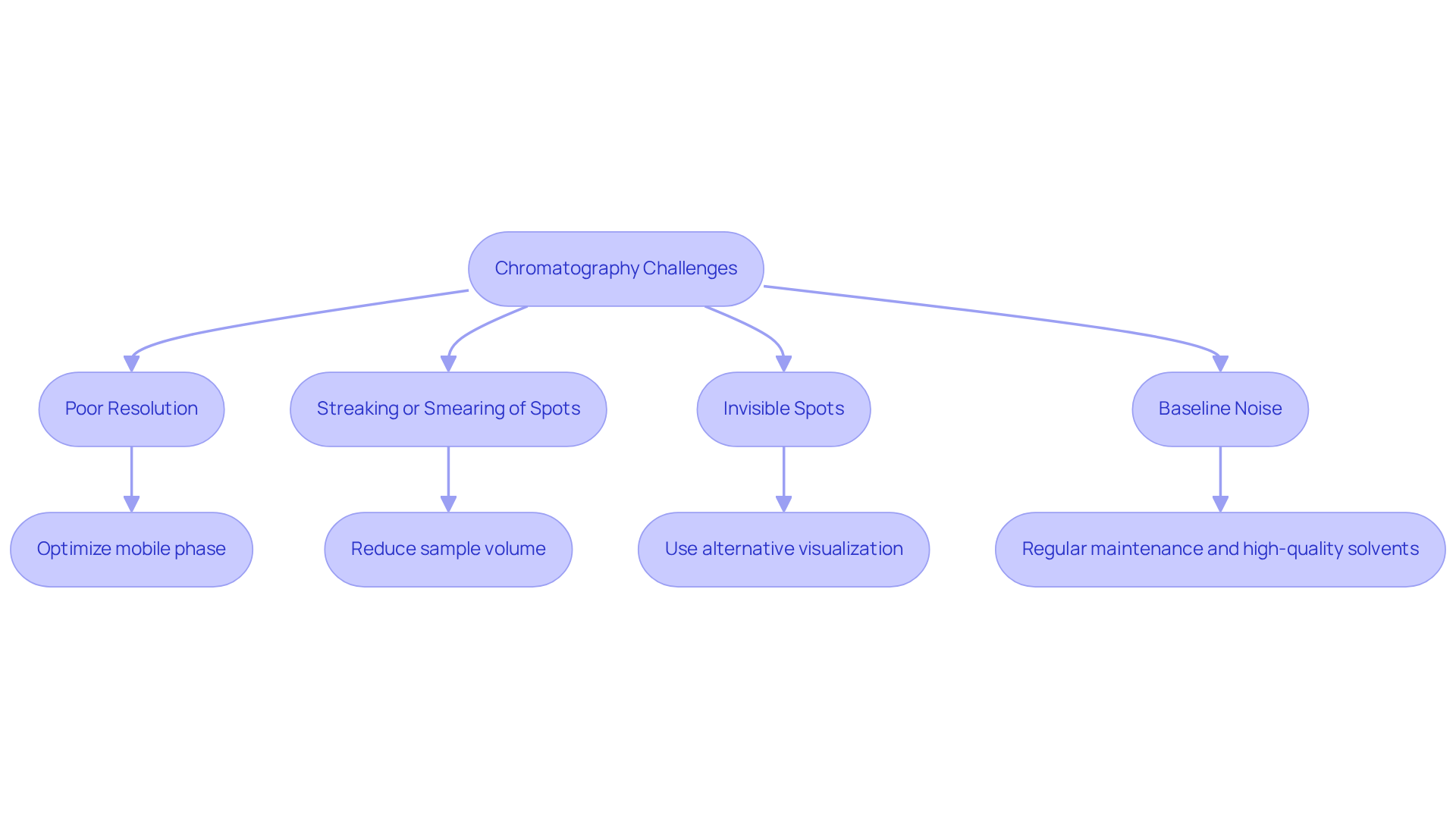
Address Challenges and Troubleshoot Common Issues in Chromatography
Chromatography presents several common challenges that can impact the quality of results.
-
Poor Resolution is a frequent issue, often stemming from improper solvent selection or insufficient development time. To address this, it is crucial to optimize the mobile phase for the specific compounds being separated, thereby enhancing resolution and accuracy.
-
Another challenge is Streaking or Smearing of Spots, which typically occurs when the plate is overloaded with sample. To mitigate this, reduce the sample volume and ensure an even application across the plate.
-
Invisible Spots can also pose a problem; if spots remain unseen after development, consider employing an alternative visualization method or adjusting the sample concentration to improve visibility.
-
Lastly, Baseline Noise can significantly affect result accuracy. Regular maintenance of the chromatographic system, along with the use of high-quality solvents, is essential for minimizing this issue.
By comprehensively understanding these challenges and implementing effective solutions, laboratory personnel can significantly enhance the reliability and accuracy of their chromatographic analyses.
Conclusion
Mastering chromatographic plate techniques is essential for achieving optimal results in various analytical applications. The effectiveness of these plates significantly influences the separation quality of compounds, underscoring their critical role in methodologies such as thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC). By understanding the intricacies of chromatographic plates, researchers can refine their techniques and enhance the reliability of their analytical outcomes.
This article delves into different types of chromatographic plates, including:
- Silica gel
- Alumina discs
- High-performance thin-layer chromatography (HPTLC)
- Reversed-phase discs
Each type serves specific applications, from drug development to environmental monitoring, demonstrating the versatility and importance of these tools in analytical chemistry. Furthermore, the step-by-step procedures outlined for using chromatographic plates provide a practical guide to ensure optimal performance. Addressing common challenges such as poor resolution and invisible spots equips practitioners with the knowledge to troubleshoot effectively.
In conclusion, mastering chromatographic plate techniques not only enhances the efficiency of laboratory processes but also contributes to the integrity of analytical results across various fields. By optimizing these techniques and remaining aware of potential challenges, professionals can significantly improve their analytical capabilities. Ultimately, this advancement fosters research and quality assurance in critical areas like pharmaceuticals and environmental safety. Embracing these best practices will pave the way for more accurate, reliable, and reproducible results in chromatography.




